Question
Question: The major product of the following reaction is: 
(A) 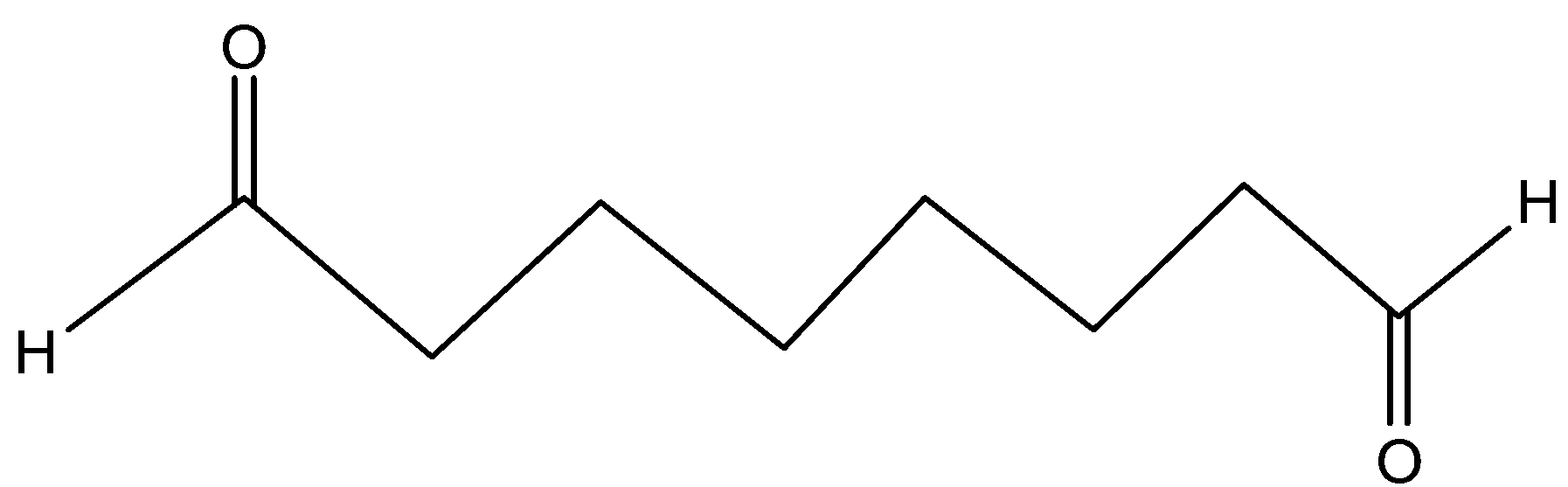
(B) 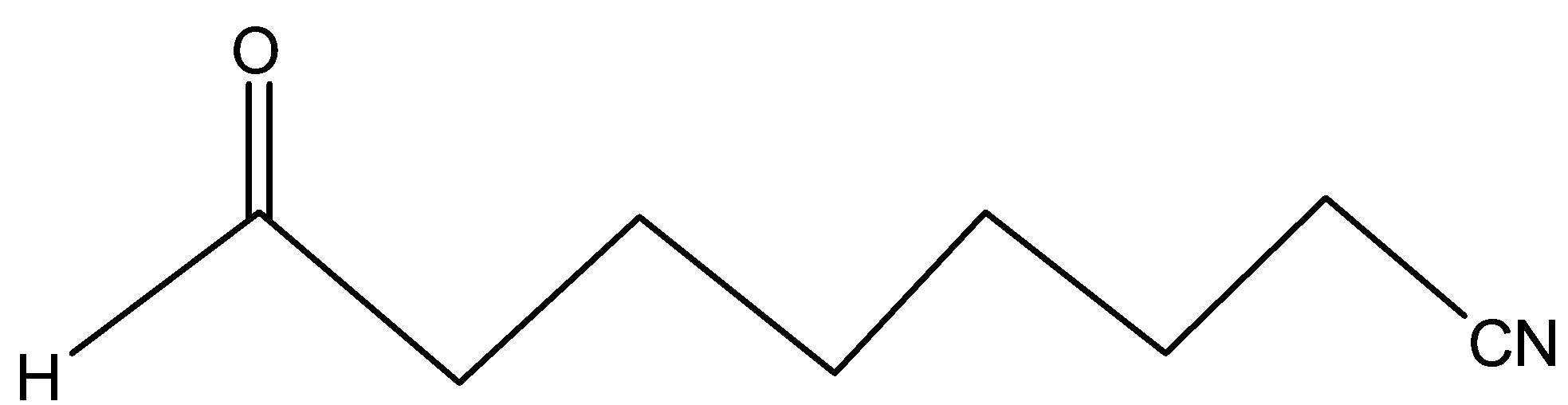
(C) 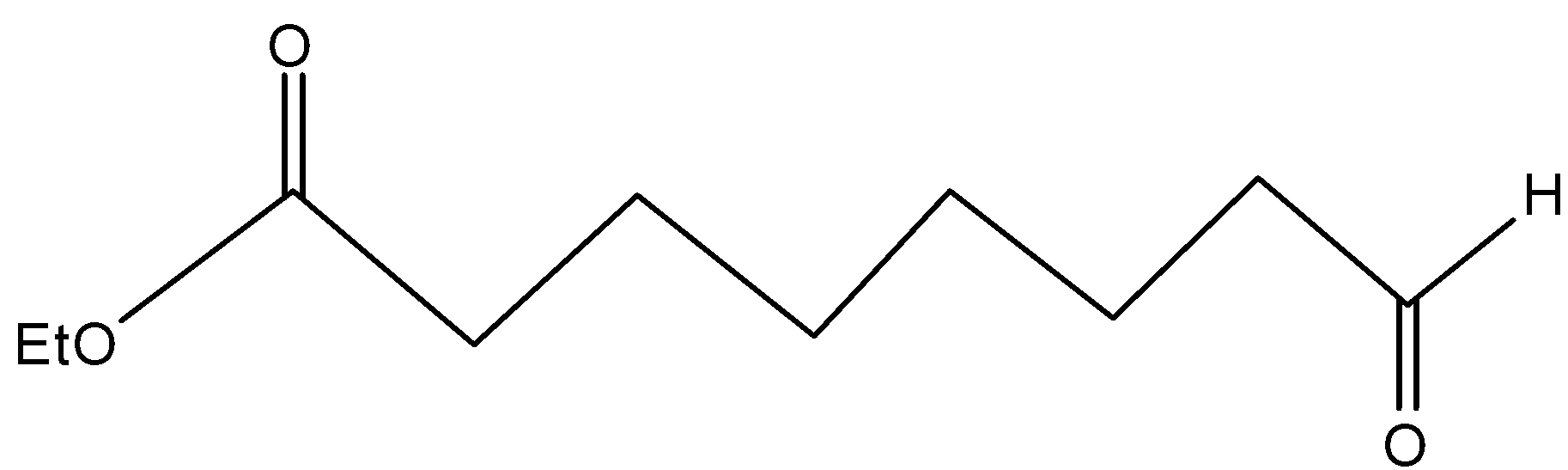
(D) 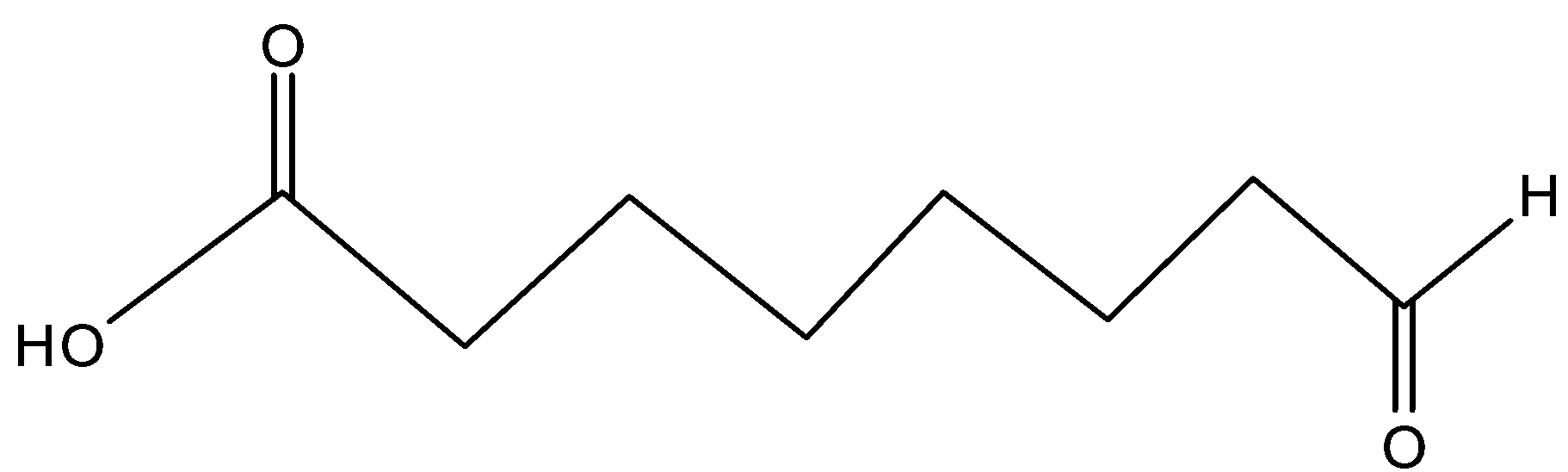
Solution
Hint The answer to this question is dependent on the fact that DIBAL – H acts as a reducing agent and it causes partial reduction of cyanide group and also the ester group and hydrolysis of this gives an aldehyde.
Complete step – by – step answer:
In the lower classes of organic chemistry, we have dealt with various topics among which the reaction mechanisms also play an important role. We have also studied about various reagents used for the oxidation and reduction reaction and some of which reduces a particular group and some reduces all the groups.
Now, let us see the role of DIBAL – H and its action as a reducing agent.
- DIBAL – H is abbreviated as diisobutyl aluminium hydride which is a reducing agent and it has the formula given as,(i−Bu2AlH)2
- This reagent has its usefulness in the organic synthesis for the variety of reduction reactions which also involves conversion of carboxylic acids and their derivatives and nitriles into the aldehydes.
- DIBAL – H is an electrophilic strong reducing agent which reduces esters into aldehydes and also reduces the other functional groups along with it such as amides, aldehydes, ketones and nitriles.
In the above given reaction, DIBAL – H reduces the cyanide group and effectively reduces the ester group along with it. The reduced product thus formed further undergoes hydrolysis to yield the respective aldehyde.
The reaction taking place is as given below,
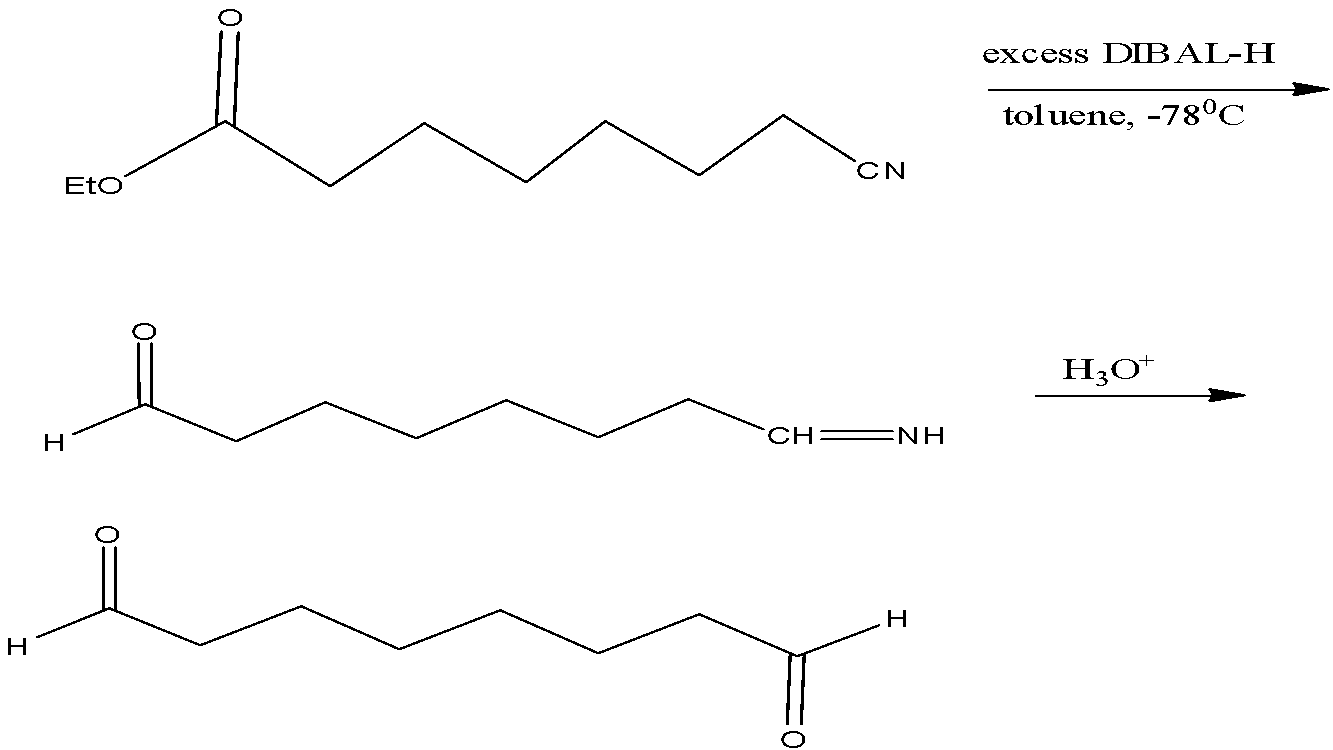
Thus, the correct answer is option A)
Note: Note that the reagent DIBAL – H efficiently reducesα−β unsaturated esters into the corresponding allylic alcohol and this reagent also reacts slowly with the electron poor compounds and also quickly with the electron rich compounds
