Question
Question: The major product of the following reaction is: 
A. 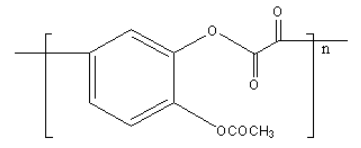
B. 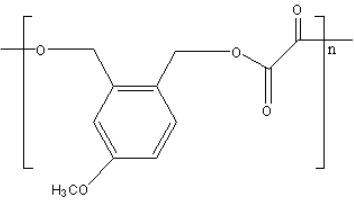
C. 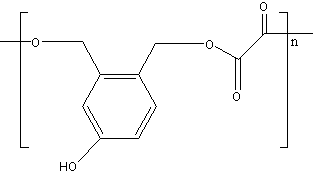
D. 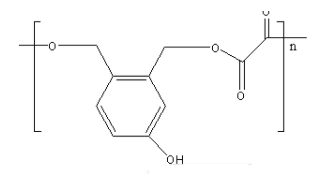
Solution
The dil. Hydrochloric acid is used to hydrolyse the ester bond to form alcohol. The diol gives polymerization with ethanedioic acid.
Complete answer:
The group[−O−CO−] is known as ester group. The compound has an ester group that can be hydrolyzed in presence of dilute acid such as hydrochloric acid.
Hydrolysis of the ester group gives the alcohol and acid.
The product of the hydrolysis reaction of the reactant with dilute hydrochloric acid is shown as follows:
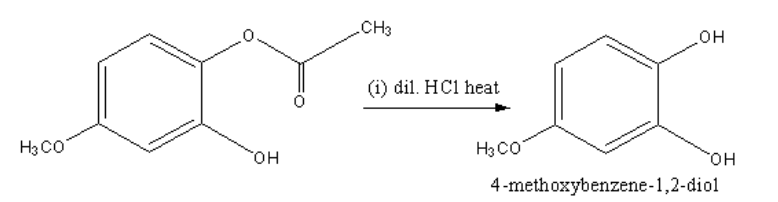
By the dissociation of the ester group of the given reactant 4−methoxybenzene−1,2−diol is formed.
The product 4−methoxybenzene−1,2−diol polymerizes in with ethanedioic acid to give the polymerized product.
The product of the reaction of 4−methoxybenzene−1,2−diol with ethanedioic acid is shown as follows:
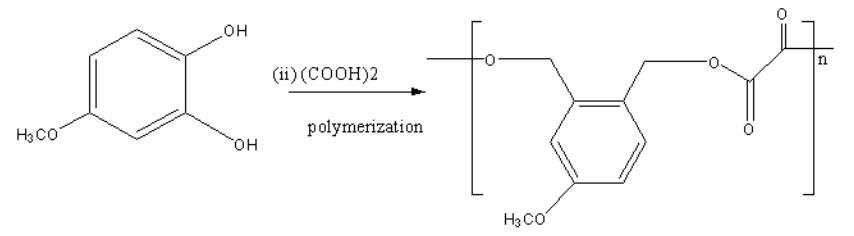
The above product is formed by the condensation polymerization method.
In condensation polymerization, −OH of reactant react with ethandioic acid as follows:
X−OH + HOOC−COOH→X−OC−COOH + H2O
Here X represents the rest of the reactant. Similarly the second −OH group of the reactant reacts with the ethanedioic acid and polymerizes.
**Therefore, option (B) is correct.
Additional information: **
Based on the mechanism of formation of product the polymerization is divided into two parts:
1. Addition polymerization: Followed by the unsaturated monomers. The bonds of unsaturated monomers break and new bonds form between monomers. No by-product is produced during the reaction.
2. Condensation polymerization: Followed by the monomers having the same or different functional groups. A by-product is formed during the reaction.
Note:
In condensation polymerization a small molecule such as water or ammonia forms as a by-product. In the reaction of 4− methoxybenzene −1,2− diol with ethanedioic acid, a water molecule forms so, the reaction follows the condensation polymerization method.
