Question
Question: The major product of the following reaction is: 
A. 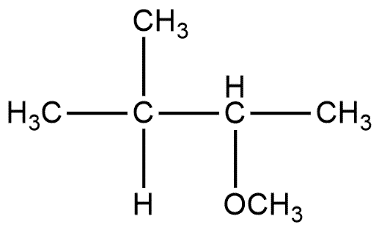
B. 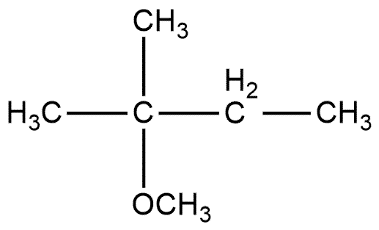
C. 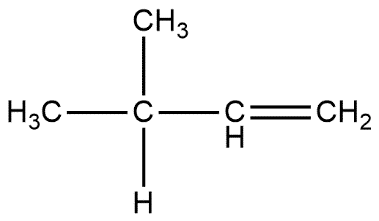
D. 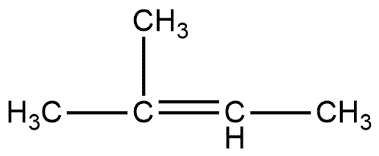
Solution
. Carbocation is an ion with a positively charged carbon atom. According to coordination numbers they can be classified into two categories: carbenium ions having coordination number 3 while those compounds which have coordination number 5 are kept in the category of carbonium ions.
Complete step by step answer:
The reaction given above is generally a step by step reaction in first step intermediate compound i.e. carbocation is formed on the 2nd carbon atom then rearrangement of carbocation is observed in next step carbon 3 becomes carbocation now and after this attack of nucleophile takes place on 3rd carbon and product is formed which is shown as follows:
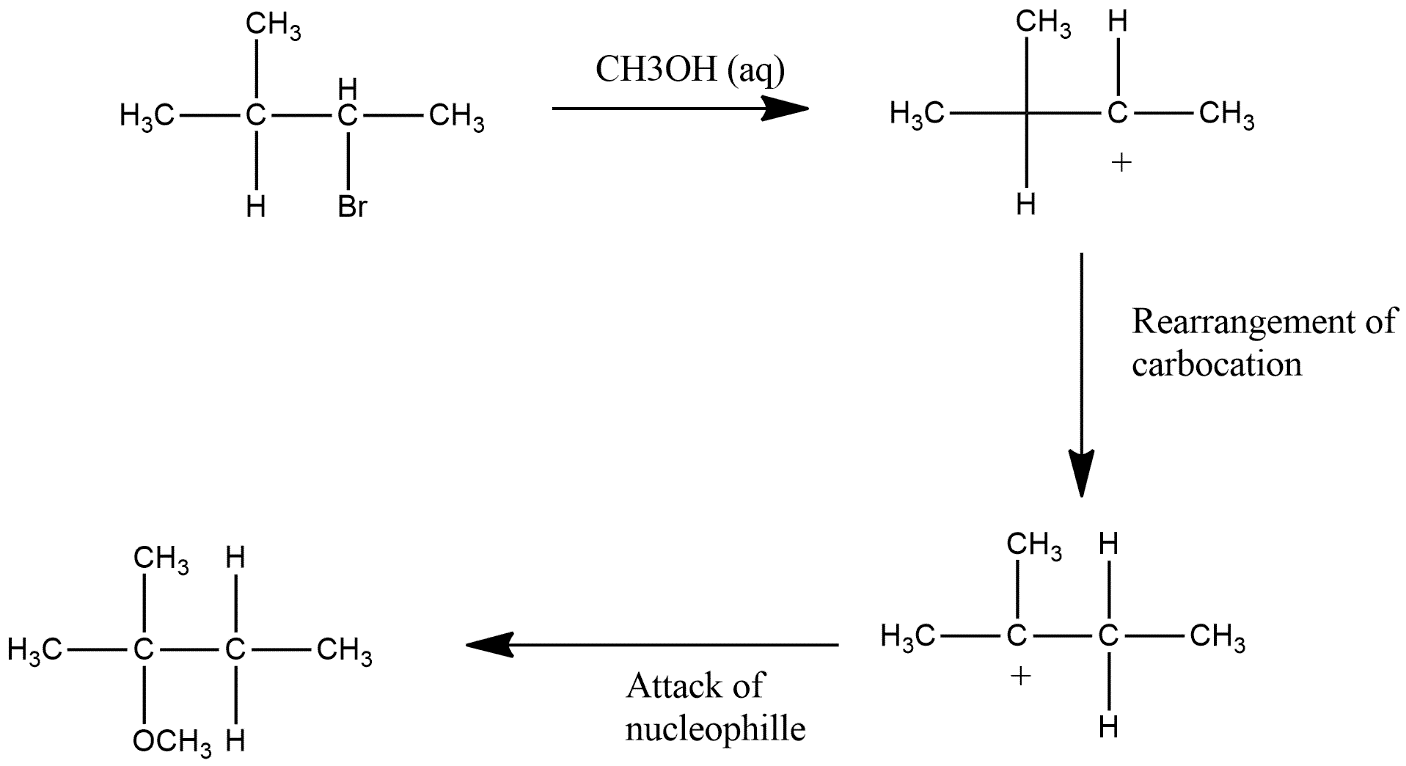
So, the correct answer is “Option B”.
Note: Nucleophile is a chemical species that donates an electron pair to form a chemical bond in relation to a reaction. All molecules or ions with a free pair of electrons or at least one pi bond can act as nucleophiles. Nucleophiles can donate electrons and keep in the category of lewis bases.
