Question
Question: The major product of the following reaction is: 
A. 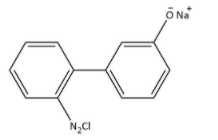
B. 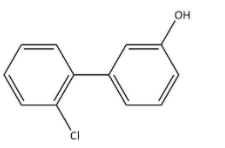
C. 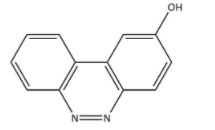
D. 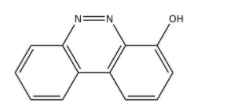
Solution
Hint: As we know that, amine is the functional group which can undergo diazotization reaction. In this reaction, a salt is formed which is a very good leaving group and when it leaves, nitrogen gas is evolved. Any reaction in which any gas is evolved that reaction helps in many ways in chemical laboratories.
Diazotization reaction is a very useful reaction because a number of products are formed by using diazotization reactions.
Aniline contains primary amine as a functional group and when it undergoes diazotization reaction, it forms an intermediate that is known as diazonium salt.
As we are given a compound which contains aniline and phenol in the presence of NaNO2,HCl so the aniline group undergoes diazotization reaction because aniline is more electron donating to the electrophile (NO) generated by NaNO2,HCl and sodium oxide is a nucleophile which replace the hydroxyl group from phenol and forms a sodium phenoxide.
The mechanism is shown in steps as follows;
Generation of electrophile- in this step NaNO2,HCl reacts to form nitrous acid which then generate nitronium ion as an electrophile by the formation of sodium hydroxide.
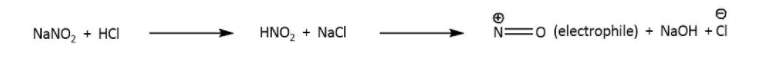
Formation of diazonium salt followed by the substitution of hydroxyl group as shown below.
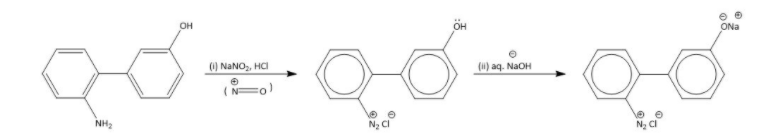
**Therefore, the correct option is (A).
Note**
The diazotization reaction is used in a number of ways to form different products with diazonium salt as a reaction intermediate. It is used for the coupling reaction with phenol and forms coloured dye. It is also used in the Sandmeyer reaction to produce alkyl halide.
