Question
Question: The major product of the above reaction is: 
(A) A hemiacetal
(B) An acetal
(C) An ether
(D) An ester
Solution
A hemiacetal is a compound in which the same carbon has an alcohol group and −OR group. An acetal is a compound in which the same carbon atom has two −OR groups. In the above reaction double bond will be replaced with −OCH2R group.
Complete step by step solution:
So, in the reactant, there is an oxygen atom that has two lone pairs present. Now the lone pair will transfer to the next bond-forming a double bond, the double bond will be attacked by the hydrogen ion because the reaction is taking place in acid. This will form a negative charge on the carbon atom next to the oxygen atom.
Now the reactant HOCH2R, the oxygen atom has two lone pairs. So the lone pair of the oxygen atom will attack the negative charge of the carbon and the −OCH2R group will be attached.
The reaction is given below:
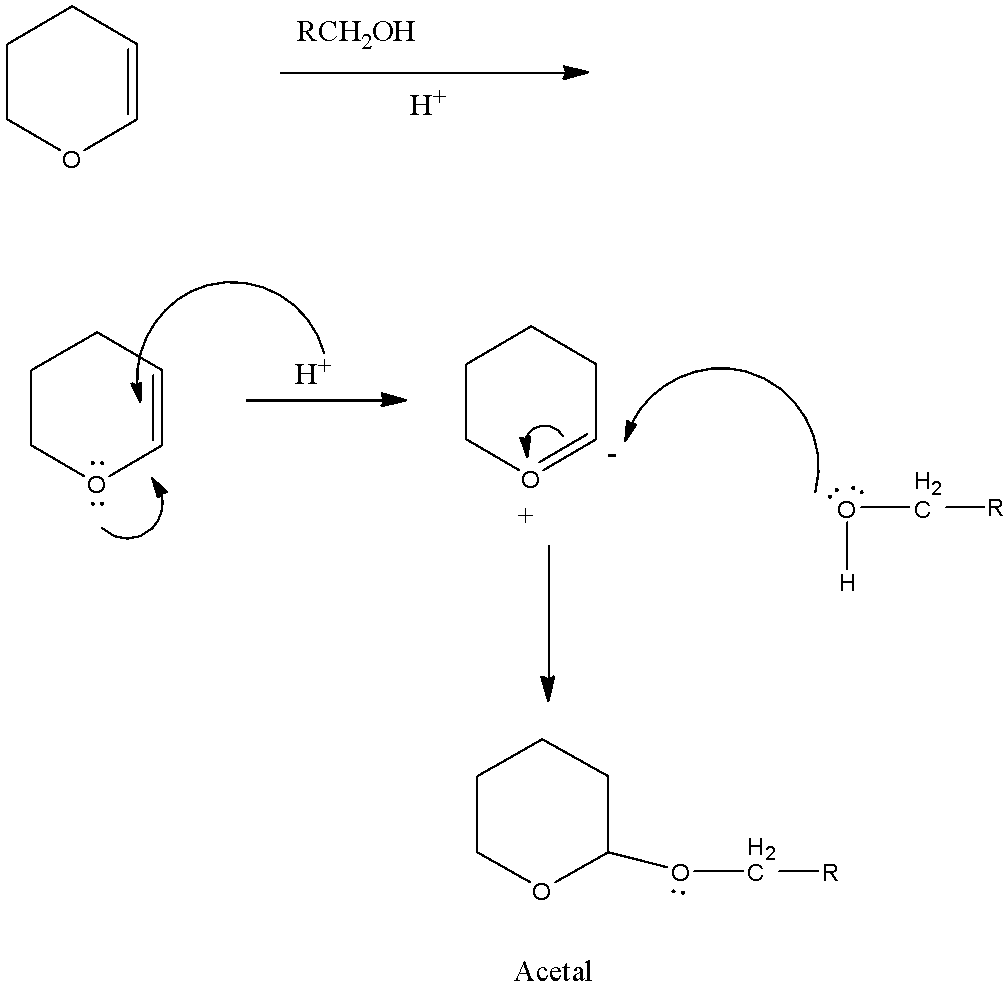
Here in this compound, the same carbon atom has two −OR groups, therefore, it is an acetal.
Therefore, the correct answer is an option (B) An acetal.
Additional information:
A hemiacetal is a compound in which the same carbon has an alcohol group and −OR group. Ether is a compound in which two alkyl groups are joined by one oxygen atom. An ester is a compound in which the functional group between two alkyl groups is −COO−.
Note: In an acetal compound the R group can be the same or different, but it must be on the same carbon atom. The above reaction can only take place in the presence of acid.
