Question
Question: The major product obtained in the reaction of aniline with acetic anhydride is? (A)  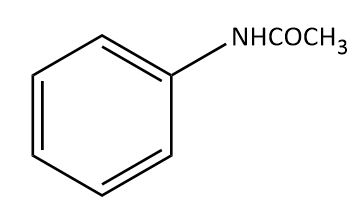
(B) 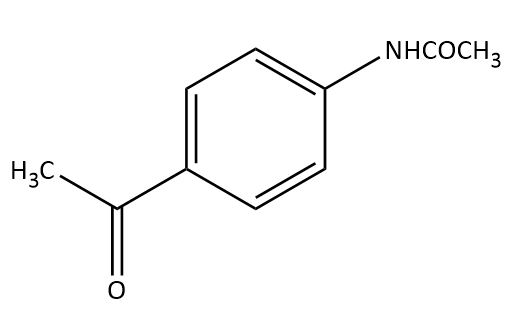
(C) 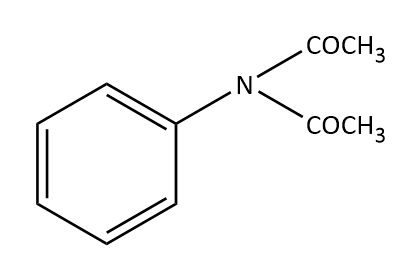
(D) 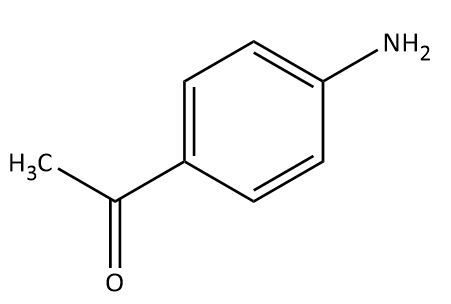
Solution
As we know that acetic anhydride contains two acetic groups bonded with oxygen and the carbonyl stretching frequency for this group is measured maximum. Therefore, it can be attacked by any group which is a Lewis base.
Complete step by step answer:
The aniline has amine group as a functional group which contains nitrogen bearing a lone pair of electrons due the amine group aniline is a very reactive for the halogenation. The nitrogen of amine has lone pair of electrons and delocalized its electron in the benzene ring by donating its electron to empty π∗orbital of benzene. Due to the delocalization the electron density on the benzene ring is increased at ortho and para positions which causes formation of trisubstituted product on benzene.
To decrease the delocalization effect on benzene, aniline reacts with acetic anhydride. The reaction is followed by an addition-elimination mechanism. The acetic anhydride group containing carbonyl carbon is very electrophilic in nature, so the amine group of aniline attacks readily on the acetic carbonyl carbon and eliminates a leaving group.
The mechanism is shown as-
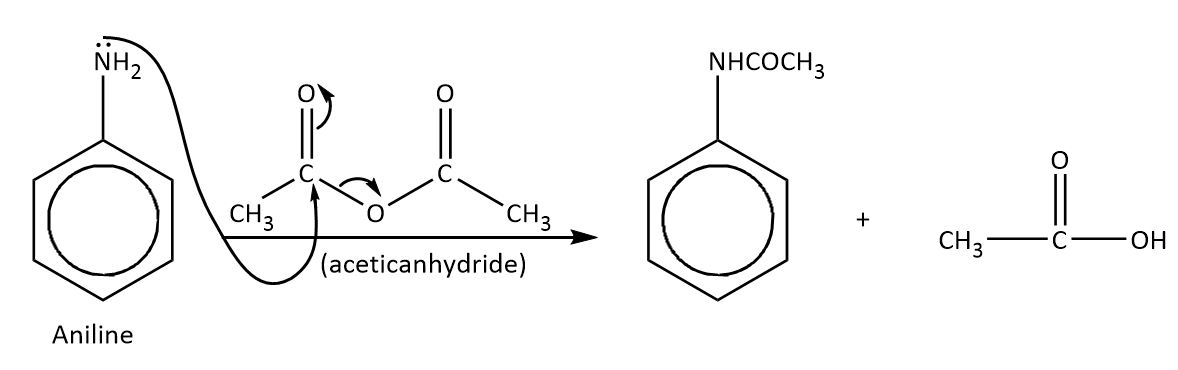
Thus, the correct option is A
Note:
Due to the partial delocalization of electron (resonance) in acetic anhydride the carbonyl group has maximum stretching frequency.
