Question
Question: The major product in the following reaction is: 
Solution
First when the Grignard reagent acts as catalyst the ketone group will be attacked changing into a complex. Then with the help of aqueous acid, the chlorine group will leave with the Grignard group.
Complete step by step answer:
First, let us see what is the reactant?
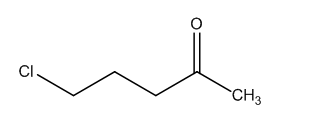
This compound is 5-chloropentan-2-one. This is the compound that comes under the ketonic group with chlorine as the substituent at the 5th place.
Now, the catalyst mentioned are (i)- CH3MgBr
This is called the Grignard reagent. They react with aldehyde, ketones, and ester to form alcohol in two steps. The first involves the nucleophilic attack of the Grignard reagent to the carbonyl group to form an adduct. The second step involves hydrolysis of the adduct with water or preferably with dilute hydrochloric acid or dilute sulphuric acid to form an alcohol.
(ii)- aqueous acid
This catalyst helps to remove the negative part of the chain-forming specific product. because acid has hydrogen ions which combine with the negative part to form the by-product.
Now when the reaction takes place,
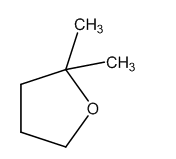
The product formed is
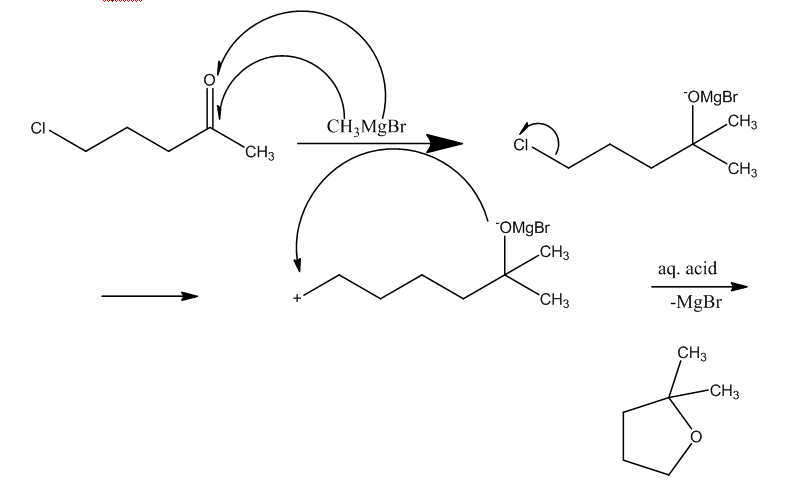
Let us understand the mechanism:
First, the Grignard reagent attacks the ketonic group, this leads to the breaking of double bond and formation of OMgBr complex. The other part of the Grignard reagent i.e. methyl group will attach to the carbon atom having the double bond.
In the next step, the chlorine bond will break which leads to acquire a positive charge on the carbon atom.
Now, the negative charge of the oxygen atom and positive charge of carbon atom will combine to form a cyclo structure.
Therefore, Option D is correct
Note: You may get confused between option (b) and (d) because in the catalyst Grignard reagent is given which mainly forms the alcohol and in all the options, only option (b) contains the alcohol group. But due to the presence of aqueous acid, it will form a cyclic structure.
