Question
Question: The major product in the following reaction is: 
A. 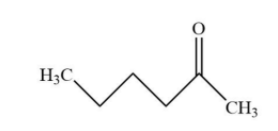
B. 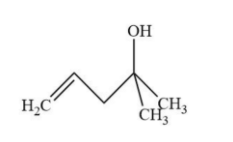
C. 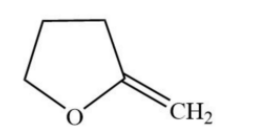
D. 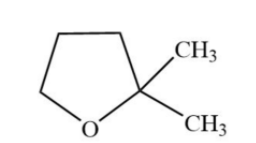
Solution
We know that CH3MgBr is known as Grignard reagent. The more electronegative an element is, the more it attracts the electron density in the bond. In the Grignard reagent, carbon of the alkyl group possesses negative charge and magnesium has positive charge.
Complete answer
Grignard reagents are made by reacting magnesium turnings with alkyl halides in ether solvents to form solutions of alkyl magnesium halide. Iodides, bromides, and chlorides can be used, as can both aryl and alkyl halides, though they cannot contain any functional groups that would react with the Grignard reagent once it is formed.
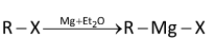
Where, R can be any alkyl/aryl while X is halogen.
Overall, it involves an insertion of magnesium into the new carbon–halogen bond. There is also a change in oxidation state of the magnesium, from Mg (0) to Mg (II). The reaction is therefore known as an oxidative insertion or oxidative addition.
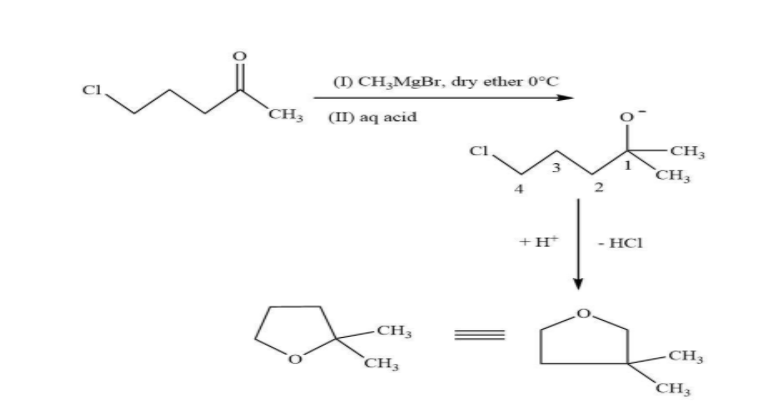
In this reaction, in the first step, the nucleophilic methyl group will attack on the electrophilic end of the carbonyl centre. Due to which a negative charge is generated on oxygen atom then in the second step there is a bimolecular substitution reaction on carbon containing chloride and chloride is removed. And overall, there is a formation of five membered compounds.
**Hence, option (D) is the correct option.
Note: **
Grignard reagents easily remove acidic protons. These strong nucleophilic reagents like Grignard reagents and organolithiums are strong bases, and may need protection from acidic protons as well as from electrophilic carbonyl groups. Among the most troublesome are the protons of hydroxyl groups.
