Question
Question: The major product formed in the given reaction is? 
A) 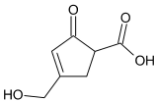
B) 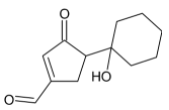
C) 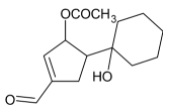
D) 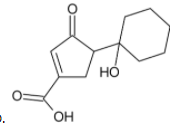
Solution
To answer this question, you must recall the action of pyridinium chlorochromate (PCC). PCC oxidises alcohols but not aldehydes or ketones.
Complete step by step solution:
PCC (Pyridinium chlorochromate) is a milder version of chromic acid which too is used as an oxidising agent. PCC oxidizes alcohols one step up the oxidation ladder to carbonyl compounds.
By the action of PCC, primary alcohols are converted into aldehydes and secondary alcohols are converted into ketones.
While chromic acid oxidizes aldehydes further to form carboxylic acids, PCC being a milder oxidising agent does not.
In the given problem, there are two alcohol groups present in the reactant. One is a primary alcohol group while the other is tertiary.
Tertiary alcohols are difficult to oxidise and their oxidation causes the breaking of a bond and removal of at-least one substituent group present on the tertiary carbon atom. Pyridinium chlorochromate is a mild oxidising agent and cannot carry out the oxidation of tertiary alcohols.
The primary alcohol group is however oxidised to form aldehyde.
Thus, we can conclude that the product formed is
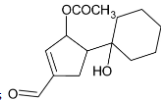
The correct option is C.
Note:
Oxidation reactions like these can be seen as a kind of elimination reaction. The carbon oxygen single bond in the alcohol is converted to a carbon oxygen double bond while hydrogen atoms are lost from both the carbon and oxygen. The elimination reaction is possible because of the presence of a good leaving group on the oxygen during the transition state, namely the chromium ion, which is eliminated when the neighbouring carbon hydrogen bond is broken.
