Question
Question: The major product formed in the following reaction is: 
A. 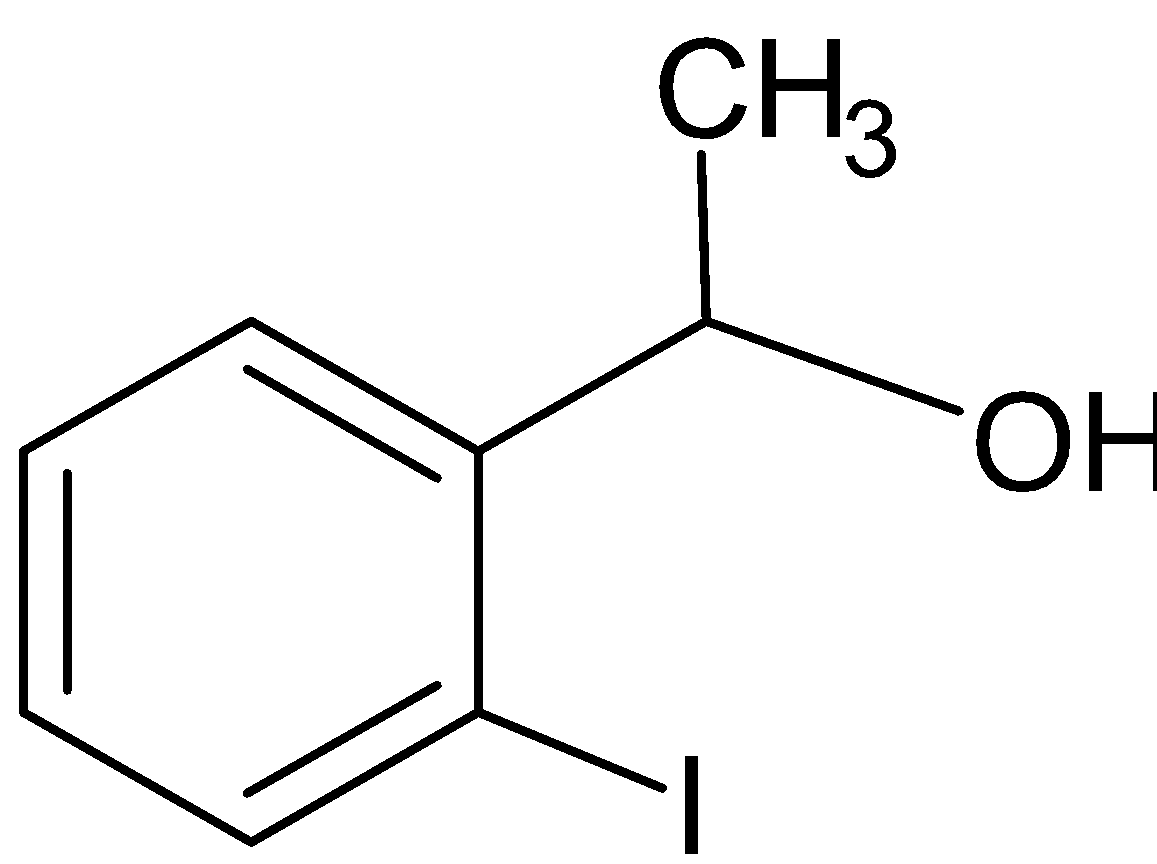
B. 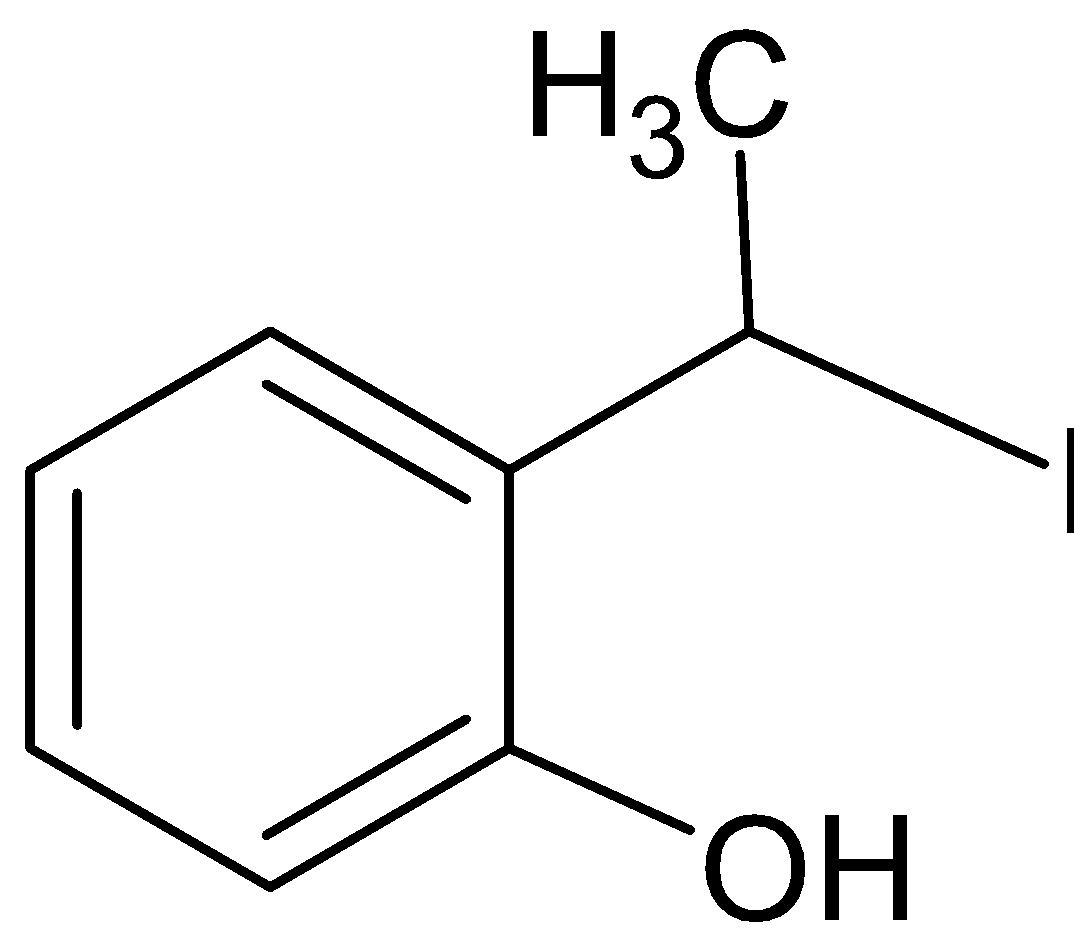
C. 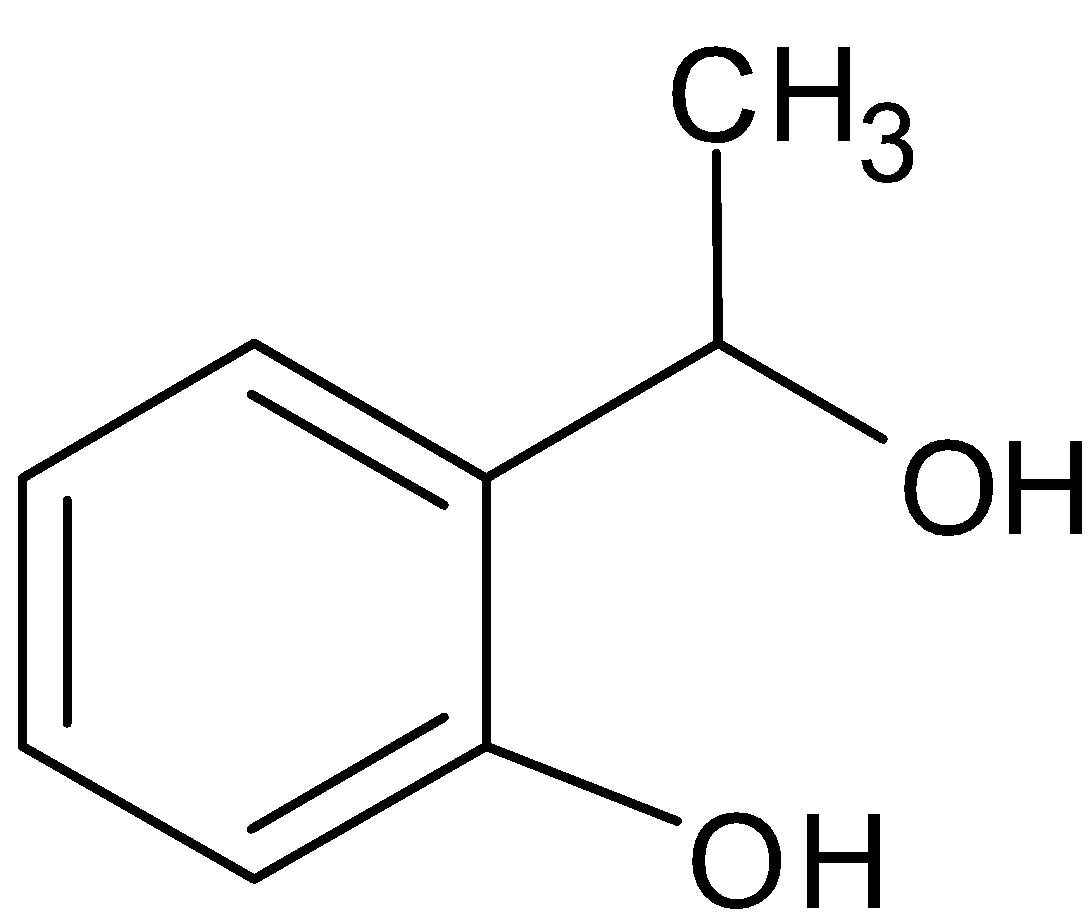
D. 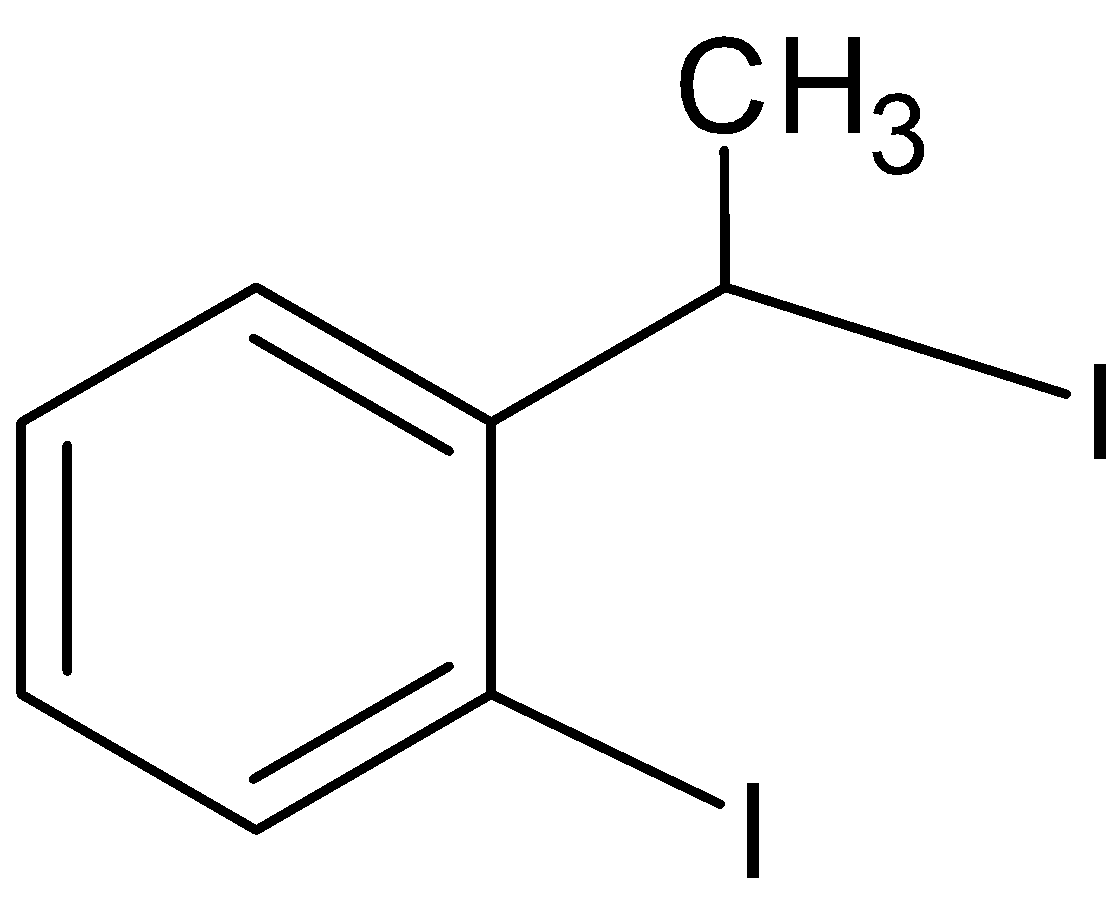
Solution
In this question we need to complete the reaction. Also we need to talk about the major product so we will look into the reaction mechanism and will solve the question.
Complete step by step solution:
Ethers are a class of organic compounds that contain oxygen between two alkyl groups. Ethers are named as alkoxy alkanes .For this question we will look into the mechanism of the reaction in the question. For the mechanism see below. From the mechanism the answer will be very clear.
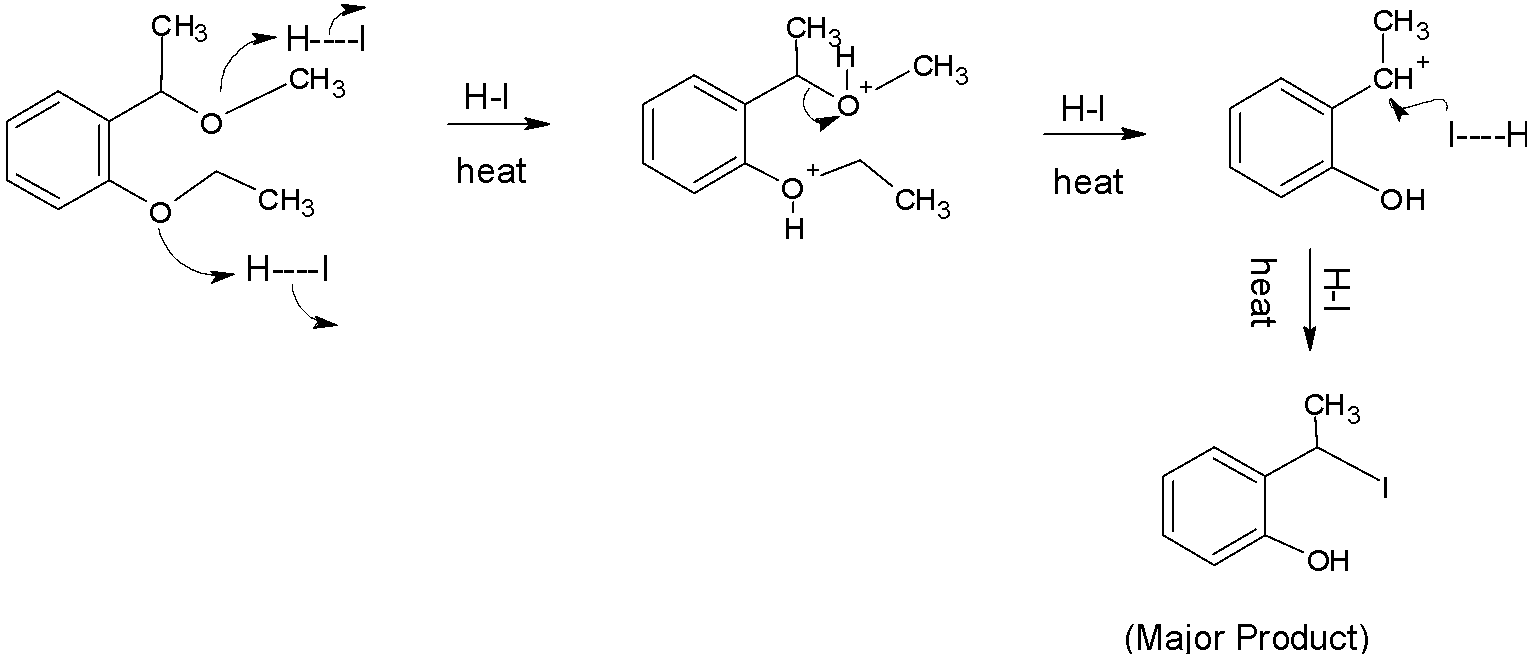
At first in the given compound the lone pairs of oxygen in both the chains attack hydrogen atoms of hydrogen iodide. Then the bond between hydrogen and iodine breaks. Both the oxygen atoms get positive charge and become an electrophile.
Then on further reacting with hydrogen iodide the carbon atom present in the upper chain becomes a electrophile and the oxygen atom in the lower chain combines with a hydrogen atom and becomes OH
On further reaction with hydrogen iodide the lone pairs of iodine attacks the carbon electrophile and it gets replaced by iodine atom.
This final product is the major product and also the answer.
The final answer is B.
Additional Information:
Hydrogen iodide is a diatomic molecule. It is also a hydrogen halide. Aqueous solution of hydrogen iodide is known as hydroiodic acid or hydriodic acid, which is a strong acid. The iodine ion has a large size due to which the negative charge is dispersed over a long space. Due to the less interaction between the constituent atoms hydrogen iodide is very reactive and hence the strongest acid of all hydro halides.
Note:
For solving this question it is a must that the reaction mechanism should be known. Without the mechanism the question will be difficult to solve. Also it should be noted that always the major product is the answer unless stated otherwise.
