Question
Question: The major product expected from the following reaction is: 
(A) 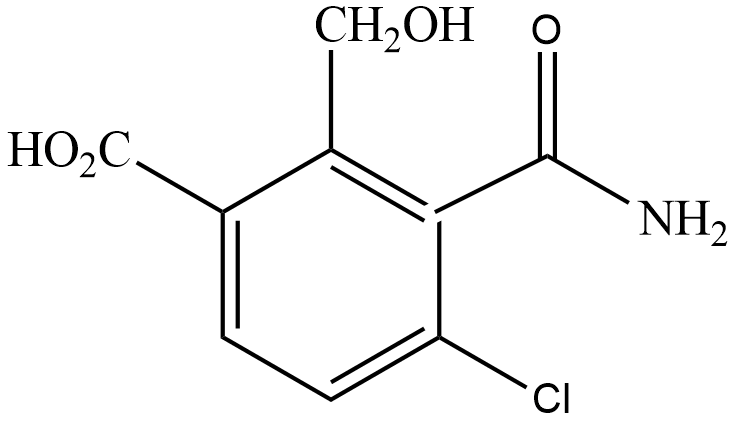
(B) 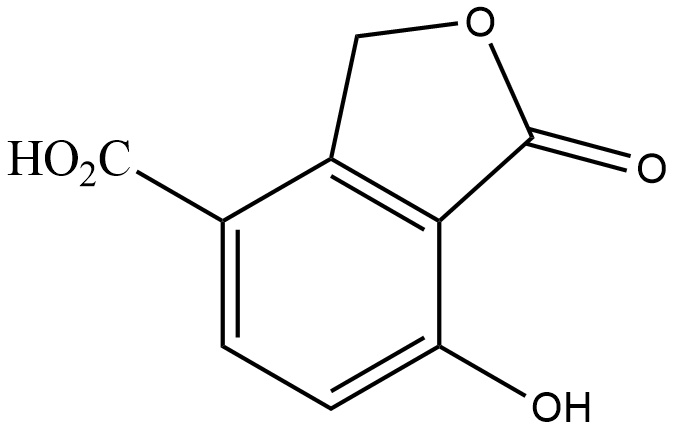
(C) 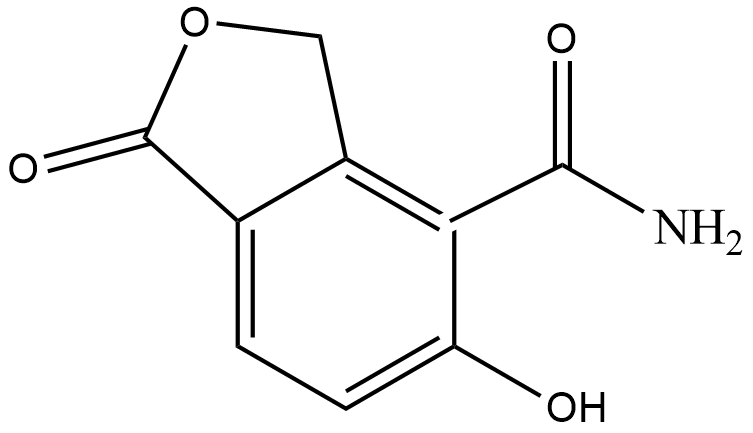
(D) 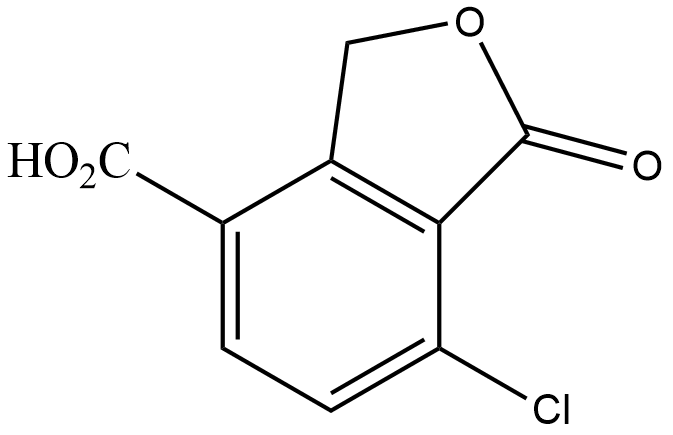
Solution
Hint : In the given compound, the functional groups carboxylic acid, methyl alcohol, amide, alcohol are attached to the benzene ring. The catalysts used here are concentrated hydrochloric acid HCl and Carbon tetrachloride CCl4 is used as a solvent. These catalysts promote the dehydration reaction in the compound.
Complete Step By Step Answer:
The IUPAC name of the given compound is 2−methanol,3−amide,4−alcoholbenzoicacid
The catalyst given for the reaction is the Hydrochloric acid HCl in the gaseous state with carbon tetrachloride CCl4 as a solvent.
The given conditions promote the esterification by the combination of carboxylic acid and alcohol.
Here, as the alcohol is not present separately in the solution, esterification of the methyl alcohol and the carboxylic acid will take place.
In the esterification reaction, first of all the hydrogen of the hydrochloric acid will get attracted to the double bond oxygen of carboxylic acid.
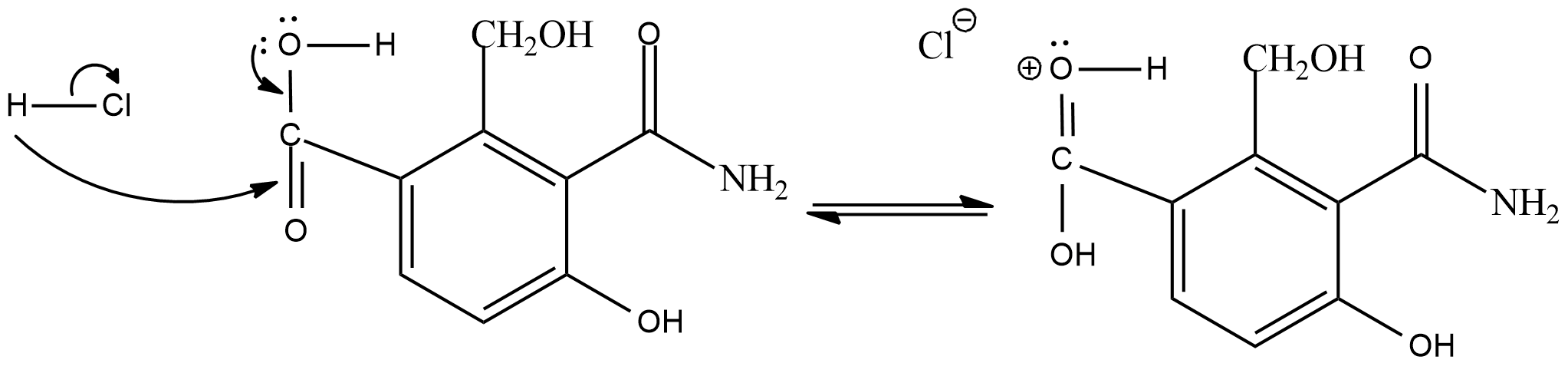
As the intermediate product will act as a electrophile, the adjacent alcohol group will act as a nucleophile and donate electrons
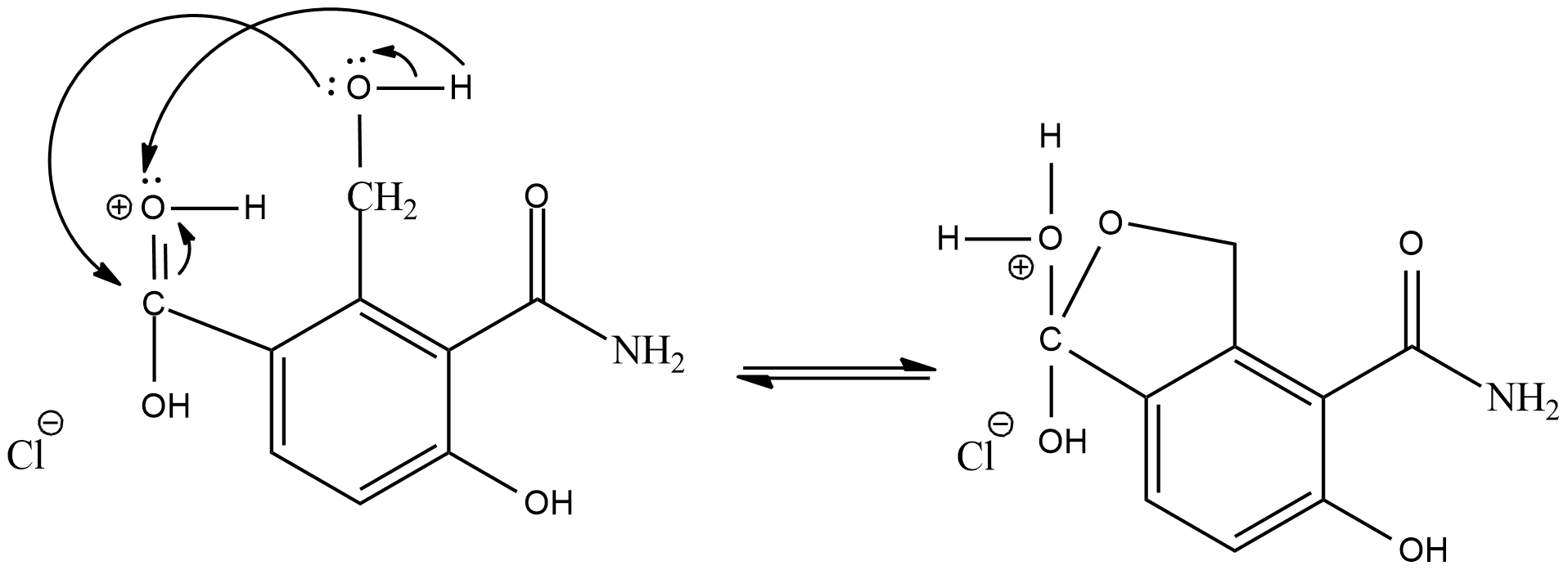
Now, the positive oxygen will leave the compound as water molecule and its valency will be balanced by the oxygen present as alcohol
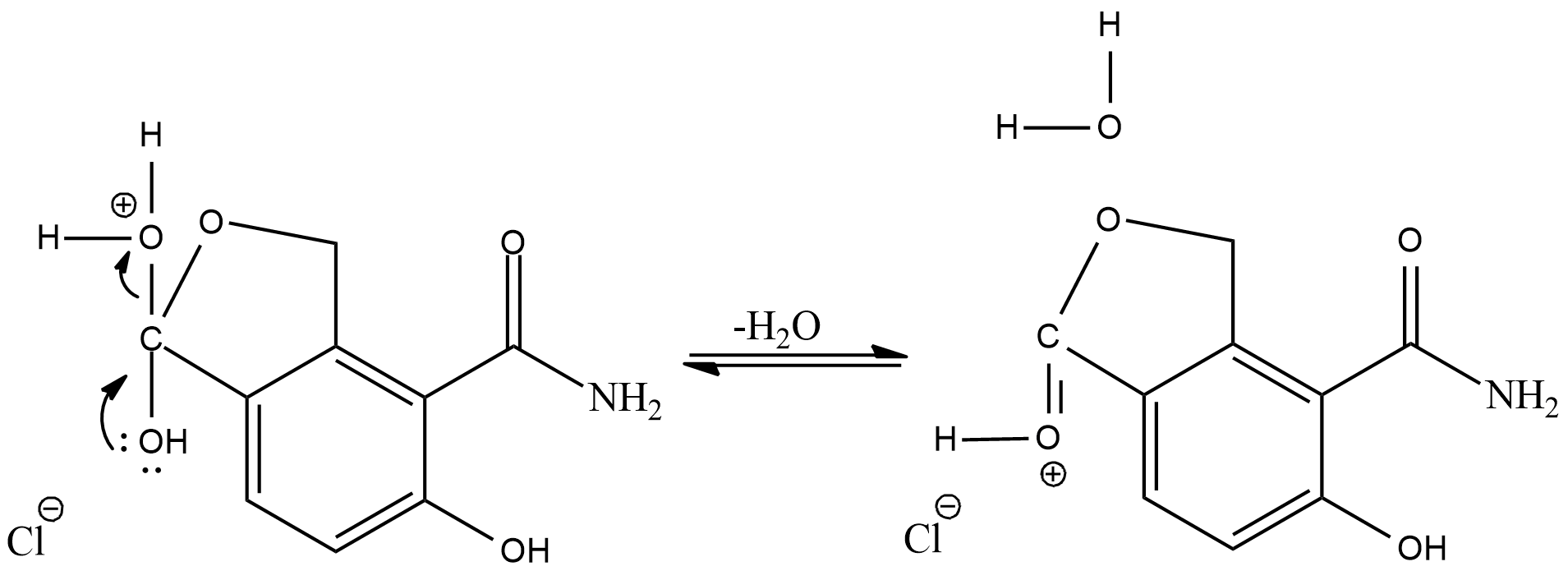
Now, the hydrogen added in the first step will again combine with chloride ion, and we will get a cyclic ester as the final product.
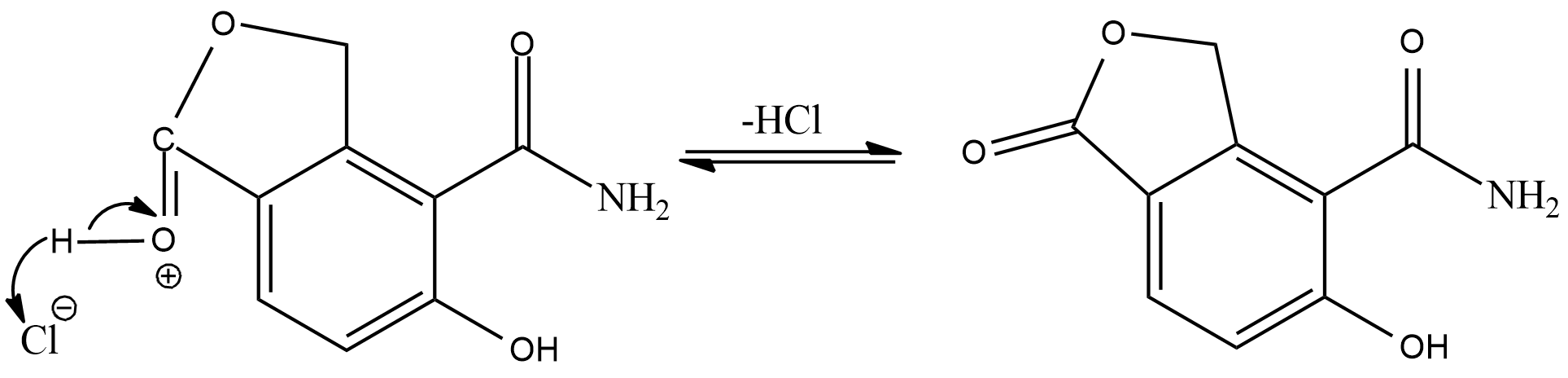
Hence, the correct answer is Option (C).
Note :
Alcohol groups show basic behavior, and on reaction with hydrochloric acid produce alkyl halides as salt. But this will not be possible in phenol, as the C−O bond possesses partial double bond nature due to resonance, and it is harder to break the bond. To convert the phenol to benzyl halide, we need to use SOCl2 as a catalyst. Also, if we need to convert the amide group to ester, we need to use CeO2 as a catalyst, which is not present here and hence, esterification of amide will not take place.
