Question
Question: The major final product in the reaction is: 
A)
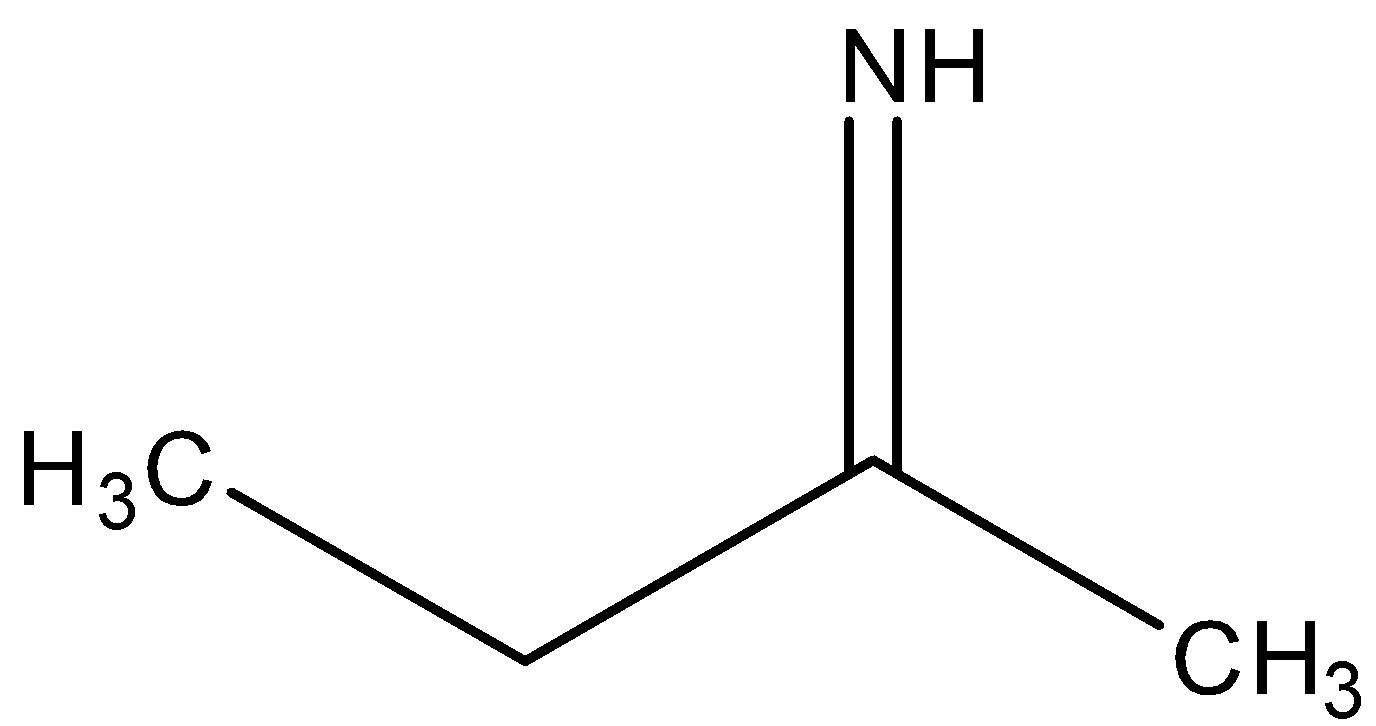
B)
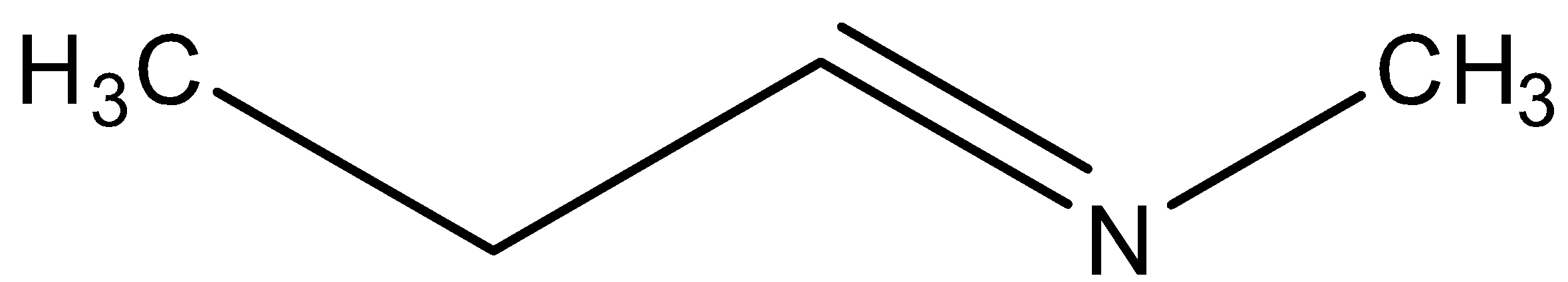
C)
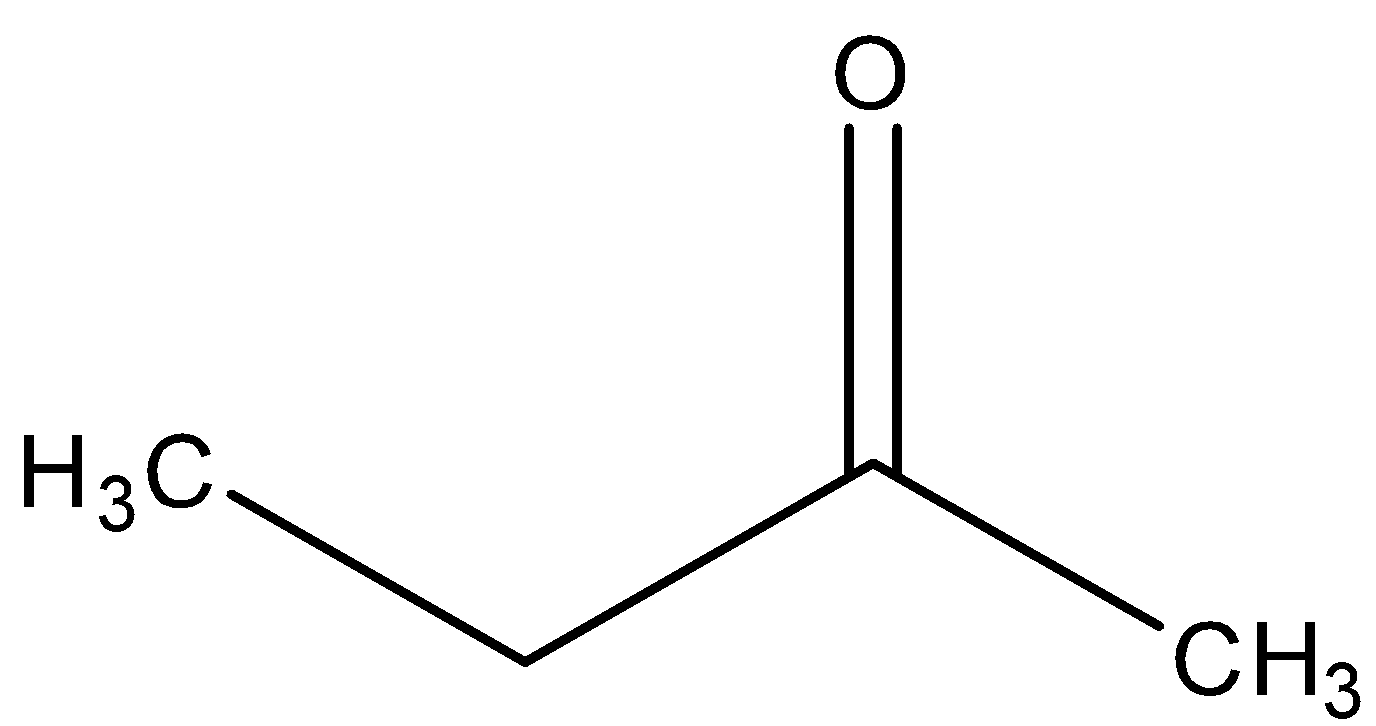
D)
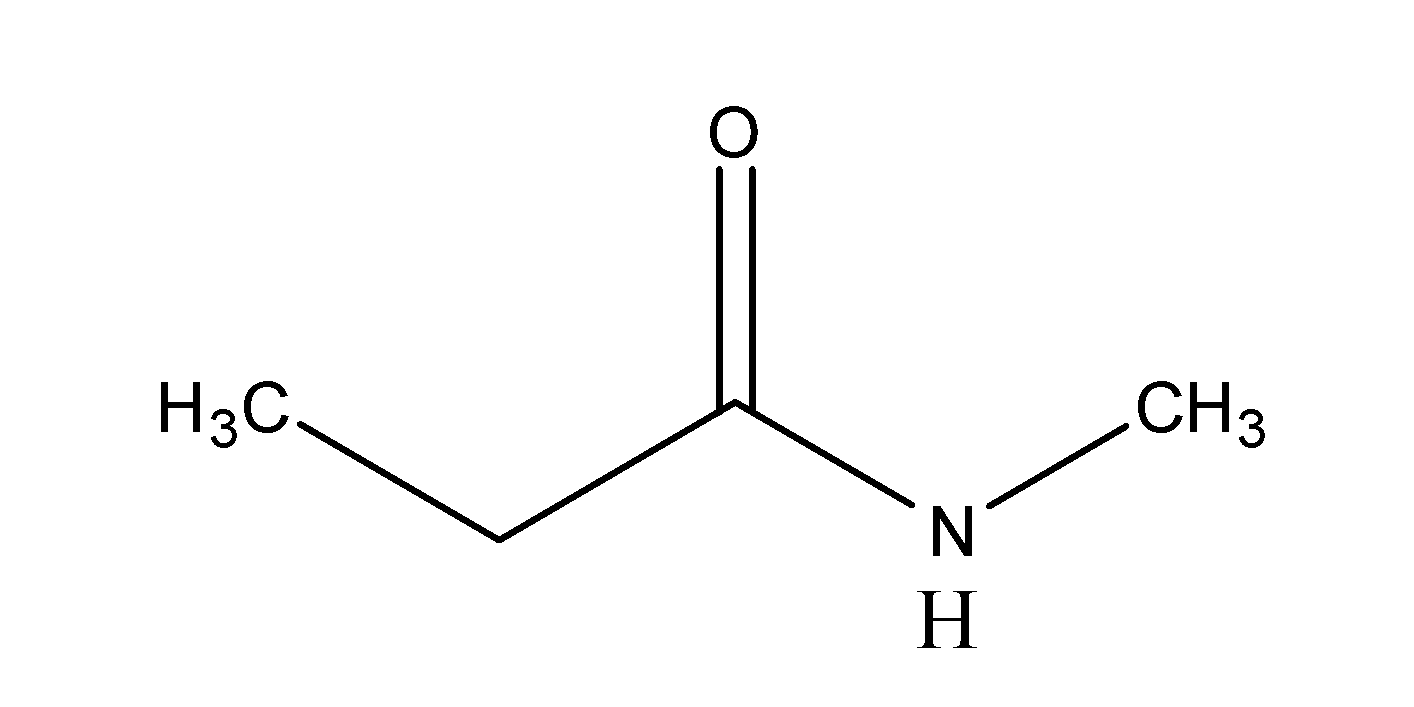
Solution
In this question we have to find the major product formed in the reaction. This a two-step reaction. The given reactant is a nitrile, and it reacts with the Grignard reagent. Here, the intermediate will react with the aqueous acid then it undergoes the hydrolysis.
Complete Solution:
Now, we have the reaction of nitrile with the Grignard reagent undergoing the hydrolysis. We will determine the product formed in this reaction step by step.
First, in this reaction the nitrile will react with the Grignard reagent. The reaction can be written as:
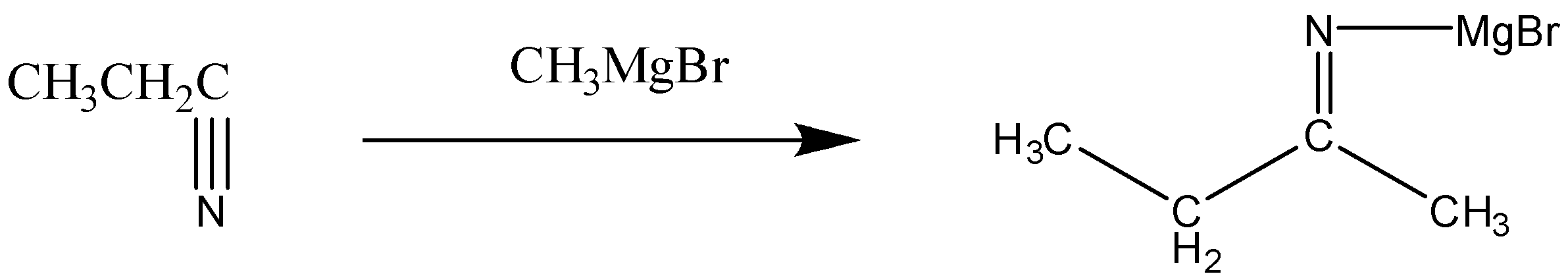
Now, in this step we can see that the nitrogen bond (C-N) is broken, and it leads to the formation of intermediate i.e. imine.
In this step, Mg Br gets attached to the nitrogen atom.
Now, this reaction will further take place accordingly in the presence of aqueous acid. The reaction can be written as:
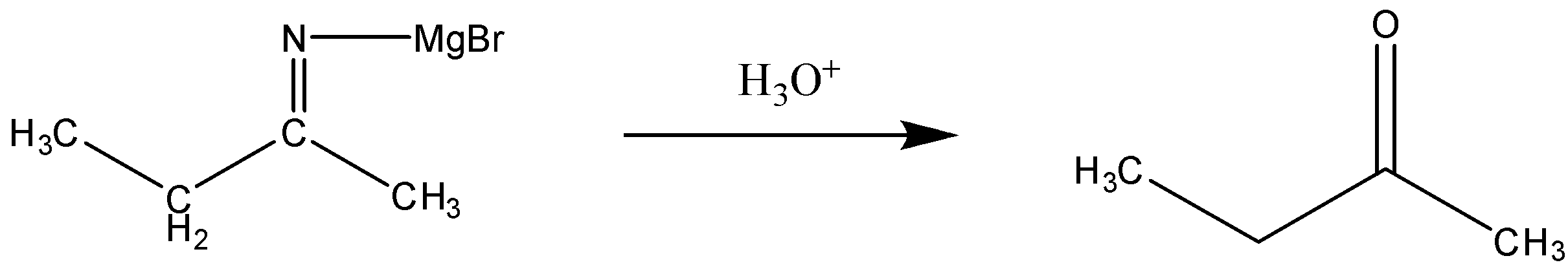
- In this step, we can see that the intermediate reacts with the aqueous acid leading to the formation of a ketone. Here, in addition magnesium salt, and the ammonia will be released.
Now, we can say that the reaction occurred as a two-step reaction.
In the last, we can conclude that the major product formed in the reaction is ketone, i.e.
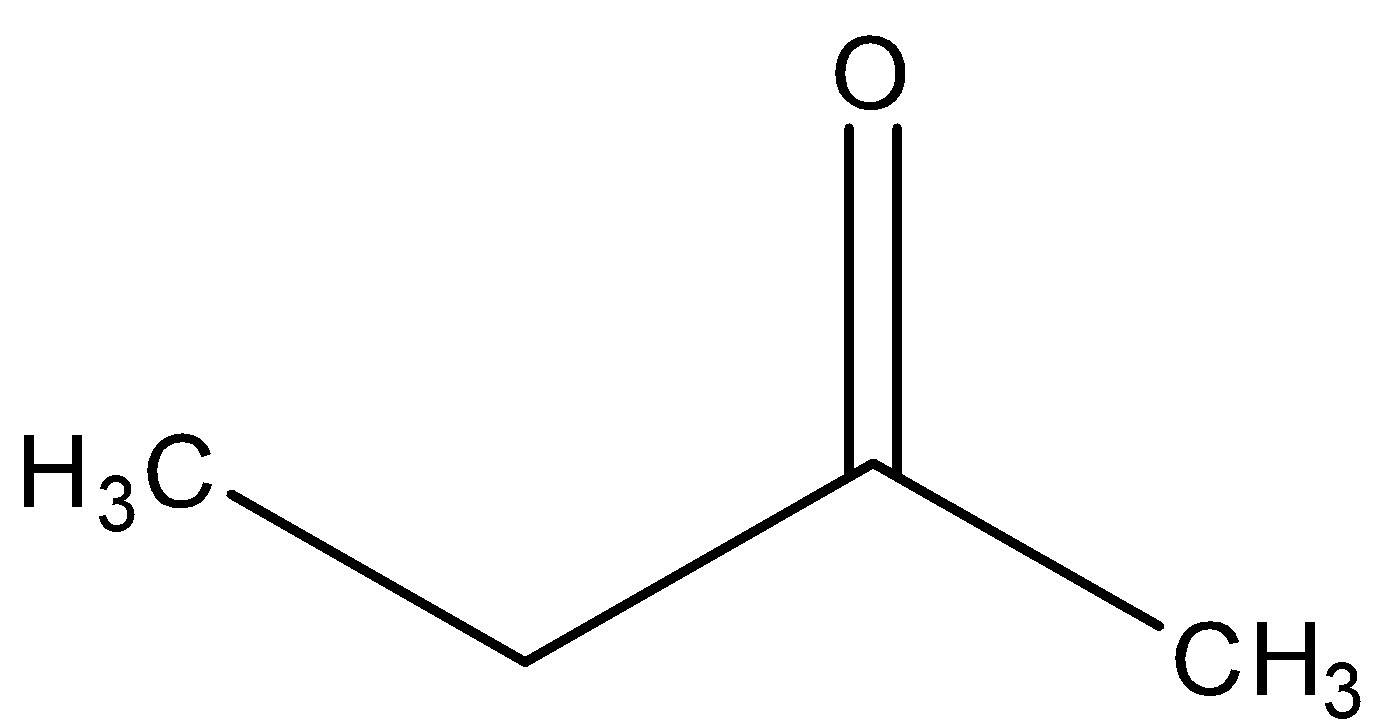
So, the correct answer is “Option C”.
Note: Sometimes what happens that in the Grignard reagent, in place of Br, any other halogen like Cl, or I is used. So, there is no need for confusion, the reaction will happen in the same way. The most important point to remember is that the reaction will happen with the aqueous acid, water, or any other solvent cannot be used; as aqueous acid is the most effective.
