Question
Question: The main reaction, \(RC \equiv CR\xrightarrow[{Lindlar's Catalyst}]{{{H_2}}}\)gives the main product...
The main reaction, RC≡CRH2Lindlar′sCatalystgives the main product as:
A.Cis-alkene
B.Trans-alkene
C.Alkane
D.None of theses
Solution
Lindlar’s catalyst is a catalyst which is used for the reduction of alkynes into alkene. The lead serves to deactivate the palladium sites, further deactivation of the catalyst with quinolone enhances its selectivity, preventing formation of alkanes.
Complete step by step answer:
Lindlar’s transformation of alkynes to cis-alkenes because of the hydrogenation occurring on the surface of metal. Both hydrogen are added to the same side of the alkyne as according to the syn- addition.
RC≡CRH2Lindlar′sCatalyst 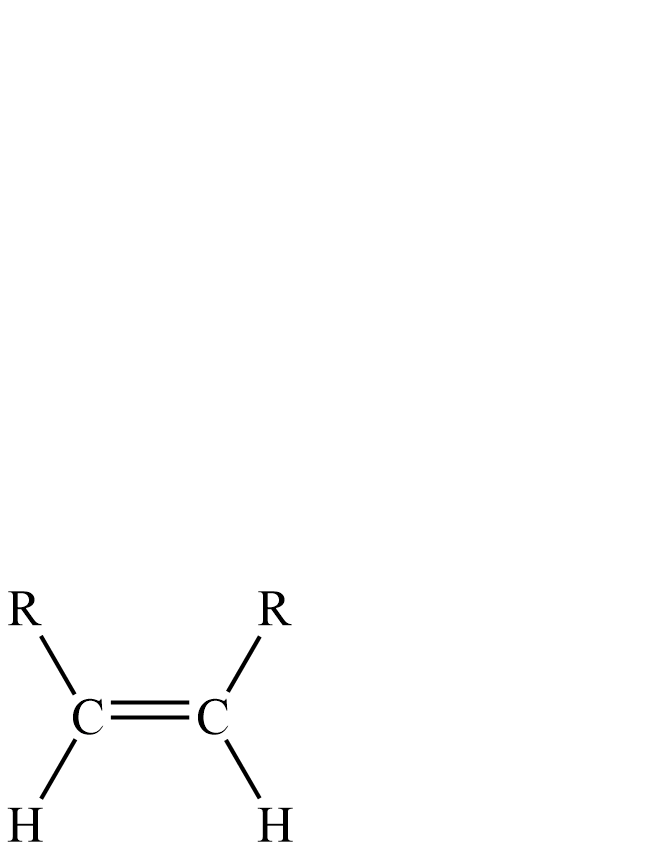
Lindlar’s catalyst has three components: palladium-calcium carbonate, lead acetate and quinoline. The quinoline prevents complete hydrogenation of alkyne to an alkane.
Now if we talk about syn-addition, it is an Addition reaction in which all new bonds are formed on the same face of the reactant molecule. As a result of the syn-addition there is decrease in bond order but increase in number of substituents.
Hence our answer is option A.
Note:
Addition reaction: Addition reaction is defined as that reaction in which two or more reactants combine together to form a single product. Addition reactions are limited to molecular compounds that have multiple bonds.
