Question
Question: The magnetic moment of \({{\left[ Ni{{X}_{4}} \right]}^{2-}}\) ion is found to be zero. Then the met...
The magnetic moment of [NiX4]2− ion is found to be zero. Then the metal of the complex ion is (X=monodentate anionic ligand).
(A) sp3 hybridized
(B) spd2hybridized
(C) dsp2 hybridized
(D) d2sp hybridized
Solution
The charge on the nickel atom should be found first. Then from molecular orbital theory we could find how many vacant orbitals are needed for ligands. By arranging the electrons in orbitals according to the details in question, we will get the hybridization of ions.
Complete step by step solution:
-The given complex is [NiX4]2−.It is given that X is a monodentate ligand. We are asked to find the hybridization of the ion.
Let's start with finding the charge on nickel(x). Since X is a monodentate ligand it will be having charge of −1. There are four ligands, hence total charge on X will be −1 multiplied by four. Thus, we can find the charge on Nickel as follows
x−4=−2 x−4=−2
x=+2
Therefore, nickel will have Ni2+ configuration. Hence two electrons would be lost from nickel and its number of electrons reduces to 26 from 28. Its electronic configuration can be given as,
[Ar]3d84s0. It can be diagrammatically represented by molecular orbital theory as follows,
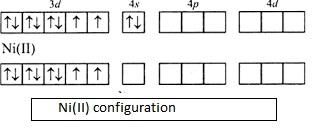
As given in the question the complex is having zero magnetic moment, the electrons should be paired. This is possible only when Ni2+ with 3d8 outer electronic configuration undergoes dsp2hybridization.
- We need four empty orbitals in order to accommodate the incoming monodentate ligands. Since the net magnetic moment is zero, the outermost electron in Ni2+ pairs with the second last electron. Now we have one vacant orbital in 3d orbital. Since we need four vacant orbitals, other ligands will occupy in the remaining orbitals. One will occupy in 4s orbital and two in 4p orbital.
- Thus, we will have the hybridization of dsp2.
Therefore, the answer is option (C).
Note: When we look at the options (B) and (D) may look similar. In spd2 hybridization, mixing of s, p and d orbitals of same electron shell takes place whereas in d2sp hybridization , mixing of s and p orbitals of same electron shell with d orbitals of another electron shell takes place. Hence, they are not the same.
