Question
Question: The lowest molecular weight alkanes, which are optically active are: A. 3-Methyl hexane B. 2,3-d...
The lowest molecular weight alkanes, which are optically active are:
A. 3-Methyl hexane
B. 2,3-dimethyl pentane
C. 2,3,3-trimethylbutane
D. 2-methyl hexane
Solution
The compounds which are going to rotate the plane polarized to either clockwise or anticlockwise then the compounds are called optically active compounds. The optically active compounds are supposed to contain a chiral carbon atom in their structure.
Complete answer:
- In the question it is given to find the optically active lowest molecular weight compounds among the given options.
- First, we should know the structure of the given alkanes to find whether they are optically active or not.
- Coming to the structure of the 3-Methyl hexane and it is as follows.
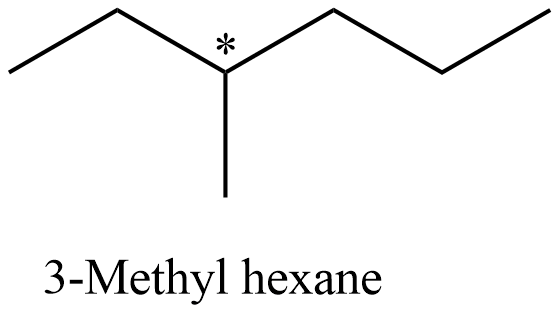
- The carbon which is marked with an asterisk symbol is optically active and the molecular weight of the 3-methyl hexane is 100.
- Coming to the structure of the 2,3-dimethyl pentane and it is as follows.
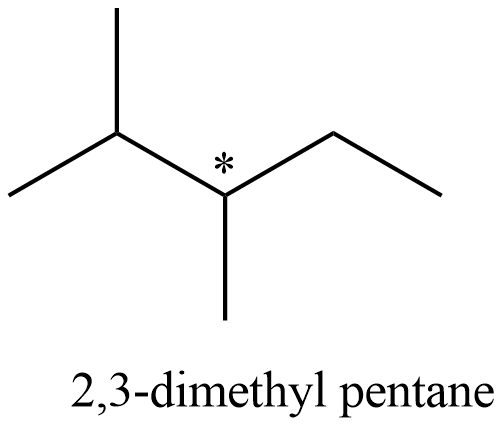
- The carbon which is marked with an asterisk symbol is optically active and the molecular weight of the 3-methyl hexane is 100.
- Coming to the structure of 2,3,3-trimethylbutane and it is as follows.
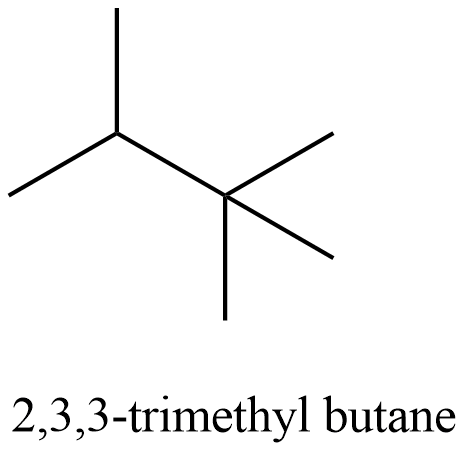
- In the above structure there is no chiral carbon then option C is incorrect.
-Come to the structure of 2-methyl hexane and it is as follows.
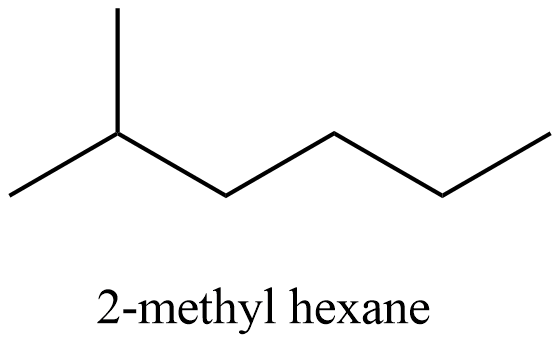
- In the above structure there is no chiral carbon then option D is incorrect.
- Therefore, the compounds that are going to have lowest molecular weight and optically active compounds are option A and option B.
So, the correct options are A and B.
Note:
The carbon atom which is going to have four different substituents attached to it is going to act as a chiral carbon and the compounds which are going to have a chiral carbon are optically active in nature when compared to the compounds that do not have the chiral carbon.
