Question
Question: The limiting radius ratio for tetrahedral shape is A.0-0.155 B.0.155-0.225 C.0.225-0.414 D.0...
The limiting radius ratio for tetrahedral shape is
A.0-0.155
B.0.155-0.225
C.0.225-0.414
D.0.414-0.732
Solution
Tetrahedral carbon is basically a carbon atom with four attachments and the carbon atom uses orbitals to achieve this geometry. Basically the radius ratio is referred to as the ratio of cation to the ratio of anion.
Complete step by step answer:
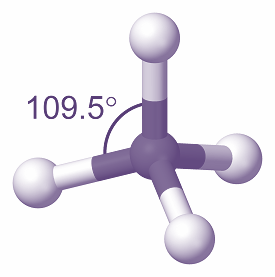
The tetrahedral is a molecular shape that is formed when there are four bonds and no lone pairs around the central atom in the molecule. The atoms bonded to the central atom lie at the corners of a tetrahedron with 109.5∘ angles between them. Generally, the molecules with tetrahedral geometries exhibit sp3 hybridization. The shape of the molecule is as shown:
Now, moving further, the radius ratio is defined as the ratio of cation to the ratio on anion. It is basically the ratio of the radius (r) of the atom or ion which can exactly fit in the octahedral void formed by the spheres of radius R. For, trigonal voids, the radius ratio is0.155, for tetrahedral voids the radius ratio is0.225, for octahedral void the radius ratio is 0.414and for the cubic voids, the ratio is 0.732.
Therefore, the limiting radius ratio for tetrahedral structure is 0.225-0.414.
Hence, option C is correct.
Note:
The radius ratio plays a very important role in the determination of a stable structure in an ionic crystal. It also helps in determining the arrangement of the ions in the crystal structure. Moreover, it is important to note that the ionic radius differs on the basis of coordination number.
