Question
Question: The length of carbon-carbon single bond of the compounds 
is expected to increase in the order
(A) Ⅲ > Ⅱ > Ⅰ > Ⅳ
(B)Ⅰ > Ⅲ > Ⅱ > Ⅳ
(C) Ⅲ > Ⅳ > Ⅰ > Ⅱ
(D)Ⅱ > Ⅳ > Ⅰ > Ⅲ
Solution
The length of the carbon-carbon single bond depends upon the hybridization of the carbon atom. The hybridization of the carbon contains S and P characters. More will be the p percentage, more will be the length of the carbon-carbon single bond.
Complete step by step solution:
For comparing the length of the carbon-carbon single bond, we need to check the hybridization of the carbons bonded via single bonds in each option.
In sp , 50% S and 50% P character is there.
In sp2 , 33.33% S and 66.66% P character is there.
In sp3 , 25% S and 75% P character is there.
Checking each option separately,
(Ⅰ)
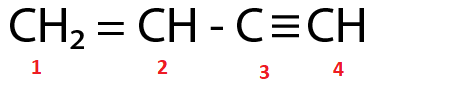
C2 and C3 are sp2 and sp hybridized respectively.
(Ⅱ)
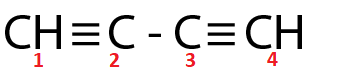
Both C2 and C3 are sp hybridized respectively.
(Ⅲ)
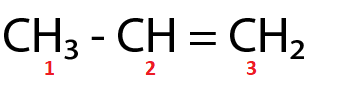
C1 and C2 are sp3 and sp2 hybridized respectively.
(Ⅳ)
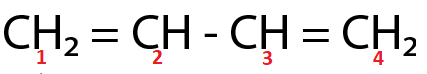 ∵
∵
Here, both C2 and C3 are sp2 hybridized respectively.
Now, we know as the p character increases, the bond length of the carbon-carbon single bond increases.
Combining the percentage p character of the carbon-carbon single bond,
For the molecule in option (Ⅰ),
Total p percentage of carbons bonded via single bond = 66.66%+50%=116.66%
( ∵ C2 and C3 are sp2 and sp hybridized respectively.)
For the molecule in option (Ⅱ),
Total p percentage of carbons bonded via single bond = 50%+50%=100%
( ∵ C2 and C3 are sp hybridized respectively.)
For the molecule in option (Ⅲ),
Total p percentage of carbons bonded via single bond = 75%+66.66%=141.66%
( ∵ C1 and C2 are sp3 and sp2 hybridized respectively.)
For the molecule present in option (Ⅳ),
Total p percentage of carbons bonded via single bond = 66.66%+66.66%=133.32%
( ∵ C2 and C3 are sp2 hybridized respectively.)
Now, we can easily compare on the basis of combined percentage p character.
The molecule in option (Ⅲ) will have the longest carbon-carbon single bond length followed by (Ⅳ),
(Ⅰ) and (Ⅱ).
Therefore, the correct order will be Ⅲ > Ⅳ > Ⅰ > Ⅱ
So, the correct option will be option C: Ⅲ > Ⅳ > Ⅰ > Ⅱ.
Additional information:
A carbon-carbon bond is a covalent bond between two carbon atoms. Single bond is the most common form: a bond composed of two electrons, one from each of the two atoms. The carbon-carbon single bond is a sigma bond and is formed between one hybridized orbital from each of the carbon atoms.
Note:
The carbon-carbon single bond length is directly proportional to the combined percentage of p of both the carbon atoms bonded via single bond and inversely proportional to the combined percentage of s of both the carbon atoms bonded via single bond.
As the combined percentage of p increases, bond length of carbon-carbon single bond increases.
