Question
Question: The leaves of a green bean plant were exposed to carbon dioxide containing the radioactive isotope o...
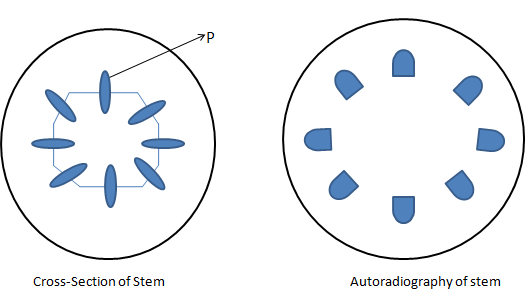
The leaves of a green bean plant were exposed to carbon dioxide containing the radioactive isotope of 14C of few hours in sunlight. A section of the plant's stem was then immediately obtained and placed on an X-ray film that blackens and was exposed to radioactivity. The following diagrams show the cross-section of the stem, and the resulting autoradiography. Why is structure P shown radioactive?
Solution
The term radioactivity refers to the self decaying property of the atom or element. Among all radioactive atoms, 14C is used for carbon dating, also called as radio dating. This technique is used to calculate the age of very old substances like metal, tree, or anything else. Any atom to be radioactive, it should be unstable in the ground state.
Complete answer:
P transported the radioactive carbohydrates from the leaves to other plant parts.
This is to understand that the basic form of food in plants is carbohydrates like Glucose, fructose, and others which break in the process of photosynthesis to give carbon dioxide, water and energy.
Though we are not sure that glucose, fructose, lactose, starch, glycogen contains that radioactive carbon so we can say that the carbon is present in the collective class of them called the carbohydrates.
So, the correct answer is “Option A”.
Note:
Carbohydrates are special types of biomolecules required for the growth and development of all the organisms on the earth. Glucose which is required to get energy by any organism comes under carbohydrates. Sugar and salts which are key food ingredients are also considered as carbohydrates only.
