Question
Question: The kinetic energy (in KeV) of the alpha particle, when the nucleus \(_{84}^{210}Po\) at rest underg...
The kinetic energy (in KeV) of the alpha particle, when the nucleus 84210Po at rest undergoes alpha decay, is
Solution
Alpha decay is the process an unstable nucleus goes under in order to become more stable. Write the equation for the alpha decay of the given particle and then find the value of energy Q, using the given table. Then apply the law of conservation of momentum to find the value of kinetic energy of the alpha particle.
Formula Used:
The equation for alpha decay: ZAX→Z−2A−4Y+24He+Q
The kinetic energies, k1+k2=Q
Complete step by step solution:
Alpha decay is a process in which an unstable nucleus transforms itself into a new nucleus by emitting an alpha particle i.e., a helium nucleus24H.
Since anα−particle has two protons and two neutrons, so after the decay, the parent nucleus is transformed into a daughter nucleus with a mass number smaller by 4 and the atomic number smaller by 2.
The equation for alpha decay can be expressed as follows:
ZAX→Z−2A−4Y+24He+Q
Here Q is the energy released in the process and can be determined from Einstein’s mass-energy relation which is given by:
Q=[mX−mY−mHe]c2
where mX,mY and mHe are the masses of the parent nucleus X, daughter nucleus Y, and the α−particlerespectively. The energy Q is shared by the daughter nucleus Y and the α−particle. As the parent nucleus is at rest before its decay, the α−particleare emitted with fixed energy. This energy in turn can be calculated by applying the laws of conservation of energy and momentum.
So, we know that the equation for alpha decay for the given nucleus will be given by:
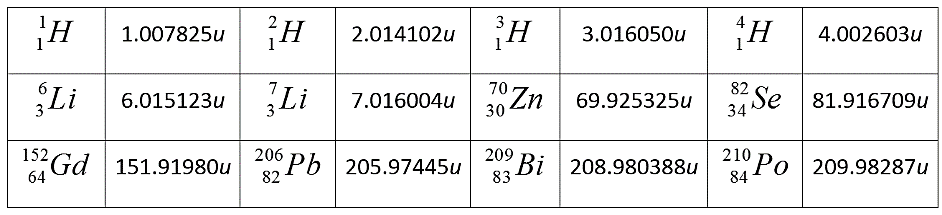
84210Po→82206Pb+24He+Q
Substituting values from the given table in the above equation, we get:
