Question
Question: The IUPAC nomenclature of the given compound is: \(C{H_2} = C(C{H_3}) - C{H_2} - C{(C{H_3})_2} - C...
The IUPAC nomenclature of the given compound is:
CH2=C(CH3)−CH2−C(CH3)2−CO−CH2−CH3
Solution
The given compound contains an alkene group as well as a ketone group. According to the IUPAC priority table, the preference of the ketone group is more than that of the preference of alkene functional group and when we name a compound which is containing a ketone functional group then the ‘one’ is used as a suffix.
Complete step by step answer:
As we can see in the given compound there are two functional groups present that are alkene functional groups that is C=C and also a ketone functional group that is R−CO−R′. In such conditions when we have more than one functional group in a compound then the compound which has higher priority according to IUPAC priority order will be named first and other groups will be considered as substituents. According to IUPAC, priority order can be given as:
Aldehyde>ketone>alcohol>alkene
As we can see that the ketone functional group has higher priority than the ethene functional group therefore, we will start numbering so that the ketone group gets the lowest number. The numbering of the compound can be given as:
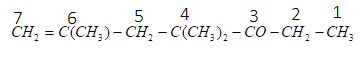
For ketone functional groups, we use ‘one’ as the suffix and for the ethene group we use ‘ene’ as a suffix. As the longest carbon chain includes seven carbons and at carbon number 4 , there are two methyl groups (we will use dimethyl) and at carbon number 6, there is one methyl group (we will use only methyl) and one ethene group. So, we will name substituent in alphabetical order. Therefore, the name of the given compound can be written as:
4−dimethyl −6−methyl−6−hepten−3−one.
Note:
Always remember that if in a compound there are more than one functional group in that compound then the functional group with the higher priority is named first and the other groups are taken as a substituent.
