Question
Question: The IUPAC name of the product obtained by the oxidation of phenol with the help of chromic acid is _...
The IUPAC name of the product obtained by the oxidation of phenol with the help of chromic acid is ____________.
A ) cyclo - hexa - 2,4 - diene - 1,4 - diol
B ) cyclo - hexa - 2,4 - diene - 1,4 - dione
C ) cyclo - hexa - 2,5 - diene - 1,4 - diol
D ) cyclo - hexa - 2,5 - diene - 1,4 - dione
Solution
In the oxidation reaction, phenolic hydroxyl group is converted to carbonyl group. A new double bond is formed during the reaction. Three double bonds of reactant rearrange to form four double bonds of product.
Complete answer:
In the question, phenol is given as the starting material, but in the options, the product is either dione or diol. The product suggests that the starting material is hydroquinone. So instead of phenol, you start the reaction with hydroquinone.
In presence of chromic acid, hydroquinone is oxidized to benzoquinone. Two protons and two electrons are removed in the reaction. Removal of hydrogen is oxidation. Hence, hydroquinone is oxidised to benzoquinone. Removal of electrons is oxidation. Hence, hydroquinone is oxidised to benzoquinone. Hydroquinone contains two phenolic hydroxyl groups. Benzoquinone contains two carbonyl groups.
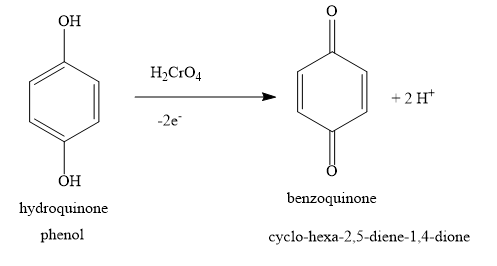
The parent compound in the product is a six membered cyclic ring. Hence, the parent compound is cyclohexane, Two carbon-carbon double bonds are present. Hence, the parent compound is cyclohexadiene. Two carbonyl groups are present.
The IUPAC name cyclo - hexa - 2,4 - diene - 1,4 - dione is incorrect. It incorrectly represents the position of the carbon-carbon double bond present in the ring. If you go by this name, then one of the carbonyl carbon will have 5 bonds. A carbon atom can have only 4 bonds. So this gives incorrect structure. The carbon atom numbered 4 has a carbon oxygen double bond and a carbon carbon double bond. It also has a carbon carbon single bond. This is not possible as a carbon atom cannot have 5 bonds. It can only have 4 bonds. Hence, the name is incorrect.
The names cyclo - hexa - 2,4 - diene - 1,4 - diol and cyclo - hexa - 2,5 - diene - 1,4 - diol are incorrect as in these names, the hydroxyl group is not oxidised. On the contrary, one carbon-carbon double bond is reduced to carbon-carbon single bond. Hence, these names are also incorrect.
The product benzoquinone contains two carbon-oxygen double bonds and two carbon-carbon double bonds. Two carbon-oxygen double bonds are represented as 1,4-dione. Here, prefix ‘di’ indicates two functional groups of the same type. The suffix ‘one’ represents a carbon-oxygen double bond. The number 1,4 represents the positions of carbon-oxygen double bonds.
Two carbon-carbon double bonds are represented as 2,5-diene. Here, prefix ‘di’ indicates two functional groups of the same type. The suffix ‘ene’ represents a carbon-carbon double bond. The numbers 2,5 represent the positions of carbon-carbon double bonds.
The parent compound is a cyclic compound and contains six carbon atoms. Hence, it is derivative of cyclohexane.
Hence, the correct option is the option D ) cyclo−hexa−2,5−diene−1,4−dione.
Note: Chromic acid is a strong oxidising agent. It oxidises alcohols to aldehdyes and ketones. Aldehydes and ketones can be further oxidised to carboxylic acids by using suitable oxidising agents. But further oxidation of ketones to carboxylic acid will result in breaking the carbon-carbon bonds.
