Question
Question: The IUPAC name of the given structure: 
A.2,2- dimethylbutane
B.isohexane
C.2,3- dimethyl butane
D.di-isohexane
Solution
IUPAC stands for the International Union of Pure and Applied Chemistry. For the nomenclature of chemical compounds, the IUPAC nomenclature in organic chemistry is a method used for the naming of organic chemical compounds. In the IUPAC system, the name of an organic compound consists of three parts:
-Word root- It is the basic unit of the name of the chemical compound. It depends upon the number of carbon atoms in the longest continuous carbon chain selected, called the parent chain. Depending upon the number of carbons in the chain the compound is assigned a word. For example, a chain containing C2 carbons will be Eth, C3 which will be a prop.
-The suffix- A suffix is added after the word root to indicate the nature of the carbon-carbon bond. For if the carbon chains contain a double bond then a suffix will be added as “ene”.
-Prefix- The groups which are not regarded as functional but present in the carbon chain as substituents are written before the word root as a prefix.
Complete step by step answer:
Let us draw the given structure in details:
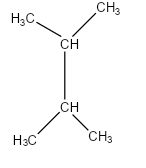
-In this structure, we can see that it is a symmetrical alkane. So if we count the longest carbon chain from any side it will be the same. The longest chain which is known as the parent chain contains four carbon atoms. In the second and third carbon, there is a methyl group attached to it.
-The IUPAC name of the given structure is 2,3- dimethyl butane.
-Hence, the correct option is (C).
Note:
-2,3-dimethyl butane is an isomer of hexane. It is a colorless liquid that boils at 57.9∘C.
-It is used in organic synthesis, as a catalytic agent, and petrochemical additive.
-The density of 2,3-dimethyl butane is 662mgmL−1
