Question
Question: The \(IUPAC\) name of the following compound is \[3{{ - (trichloromethyl) benzoic acid}}.\] benzoicacid.
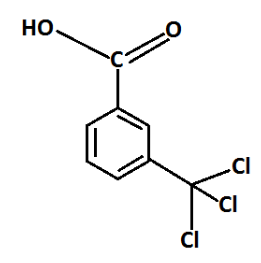
A. True
B. False
Solution
In the given compound we have three functional groups namely, carboxylic acid,methyl and chlorine one of the functional groups is chosen as the principal functional group and the compound is then named on that basis. The remaining functional groups which are subordinate functional group .The order of decreasing priority for functional group is: −COOH , −SO3H , −COOR , COCl , −CONH2 , −CHO ,−CO ,−OH
Complete step-by-step answer: In the given question the principle characteristic of the compound is carboxylic acid. This principal characteristic group is expressed at the end of a name by means of a suffix that is benzoic acid is substitutive nomenclature.
The alkyl group is attached to 3rd position. For alkyl groups the prefix ‘methyl can be used in the presence of principal higher priority group also three chlorine are also attached to methyl carbon so, The prefix can be used these chlorine ‘trichloro’.
Thus the name of the given compound is 3−(trichloromethyl)benzoicacid. So, the given IUPAC name is true.
Additional information: 3−(trichloromethyl)benzoicacidis an organic compound. Its molecular formula is C8H5Cl3O2 and the molecular mass is 239.5g/mol and the exact mass is 237.93g/mol . In the given compound, functional groups are attached to the benzene ring.
Hence, the correct option is A.
Note: The longest chain of carbon atoms containing the functional group is numbered in such a way that the functional group is attached at the carbon atom possessing lowest possible number in the chainIf the two substituents are found in equivalent position, the lower number is given to the one coming first in the alphabetical listing.
