Question
Question: The IUPAC name of the compound is 
A.2- ethyl- ethyl acetate
B.Ethyl- 3- methyl butanoate
C.Ethyl-2- methyl butanoate
D.2- methyl butanoic acid
Solution
The IUPAC nomenclature is a chemical nomenclature which is used for the naming of organic compounds by following some rules and regulations. And it is proposed by the International Union of Pure and Applied Chemistry. For solving an IUPAC name, first we have to identify the longest carbon chain present in the molecule and look if any functional is there or any branched chain is there or not. After that, conclude the name into a single word.
Complete answer:
The IUPAC name of the given compound is not 2- ethyl- ethyl acetate. Because, there is no ethyl group present in second position. Hence, option (A) is incorrect.
Ethyl- 3- methyl butanoate is not the IUPAC name of the given structure. Because, the methyl group is not present in the third position of the molecule. Hence, the option (B) is incorrect.
The IUPAC name of the given compound is Ethyl-2- methyl butanoate. Because, by following the rules of IUPAC, The longest continuous chain of carbon atom in the compound is carbon with four. Therefore, the name should contain butane. And one methyl group is present in the second position of the compound and one ethyl group is also present in the compound.
And the parent compound contains one acid group. So, the suffix should be ‘-oate’.
Let’s see the numbering of compound,
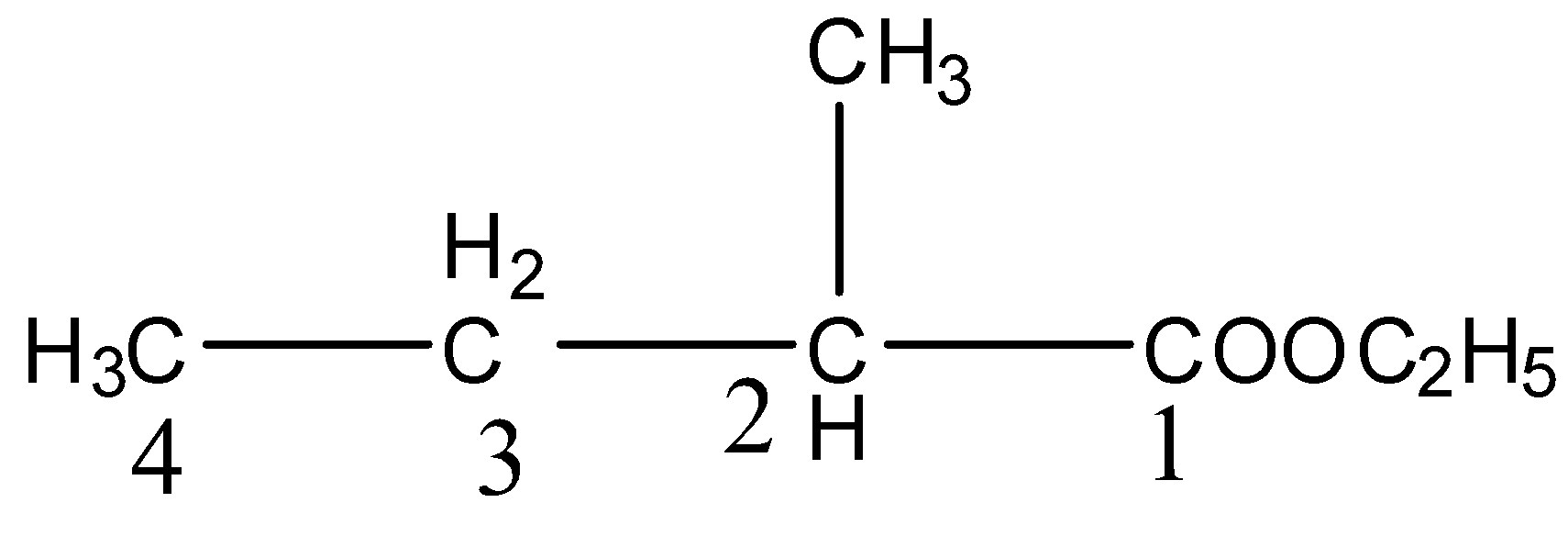
Therefore, the IUPAC name of the compound is Ethyl-2- methyl butanoate. Hence, option (C) is correct.
The IUPAC name of the compound is not 2- methyl butanoic acid. Because it does not follow the rules of IUPAC. Hence, the option (D) is incorrect.
Hence, option (C) is correct.
Note:
The IUPAC nomenclature is used for the naming of organic compounds. For that, first we have to identify the longest continuous carbon chain present in the parent compound and find out the group linked to this longest chain. The name is different for each functional group. For example, if the acid group is present in the compound, the suffix should be ‘-oate’. Like that, we are naming an organic compound.
