Question
Question: The IUPAC name of the compound is \(C{H_3} - CH({C_6}{H_5}) - C{H_2} - CH(OH) + C{H_2}C - {H_3}:\) ...
The IUPAC name of the compound is CH3−CH(C6H5)−CH2−CH(OH)+CH2C−H3:
A.1 – Ethyl – 3 – phenyl – 1 – butanol
B.2 – Phenyl – 4 – hexanol
C.5 – Phenyl – 3 – hexanol
D.5 – Benzyl – 3 – hexanol
Solution
We find the name of the longest continuous carbon chain and we should identify and name groups attached to the chain, So, for the alcohol we add the suffix "ol" and there is a phenyl group present at carbon – 5 position and the longest carbon chain is six.
Complete step by step answer:
Given in the question,
CH3−CH(C6H5)−CH2−CH(OH)+CH2C−H3
Number of Carbon atoms (C) are 12
Number of Hydrogen atoms (H) are 18
And number of Oxygen atom (O) is 1
The molecular formula is C12H18O
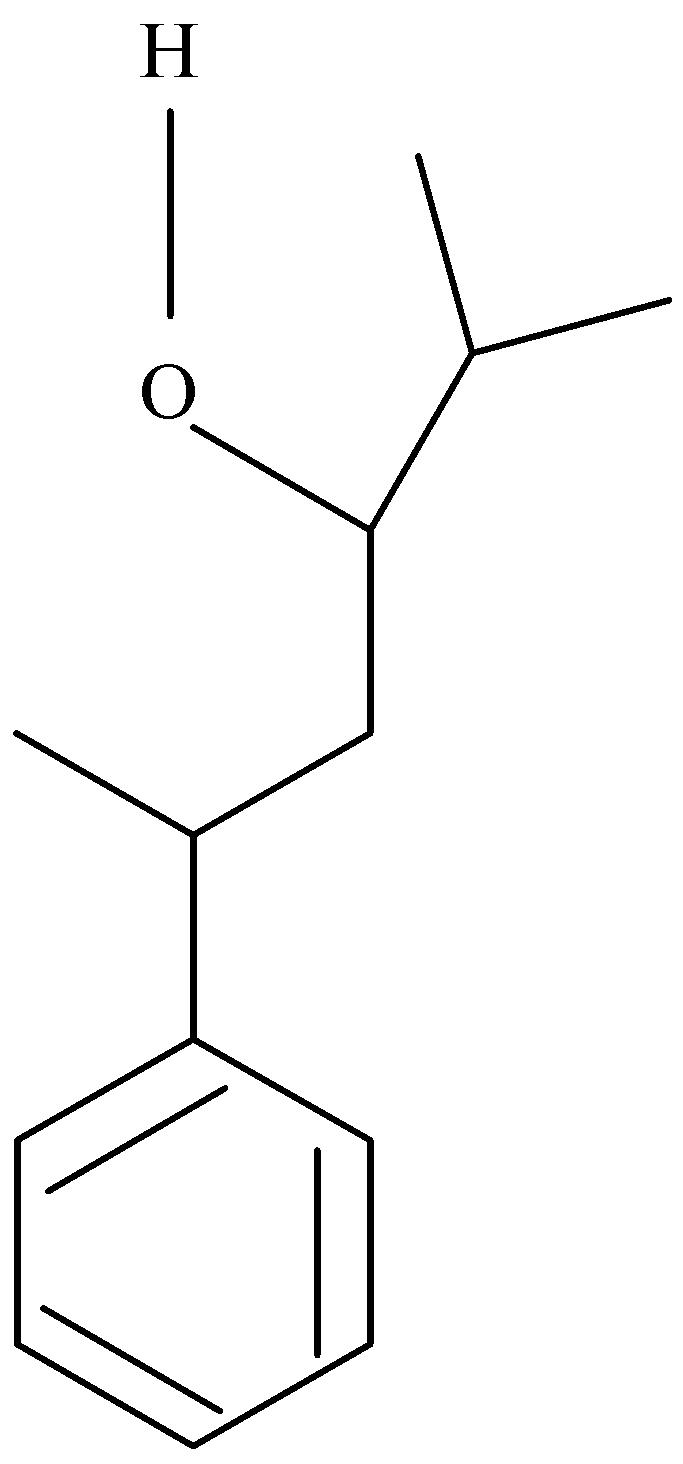
C12H18O
First of all, according to IUPAC rules, the numbering of the carbon chain should be from the left side, so that the carbon atom attached to the phenyl group gets the least number.
Longest carbon chain has to be chosen such that the functional group has the lowest number.
So, we start numbering from the carbon of the alcohol group (−OH) . Thus, there are 6 carbons is the longest chain.
At carbon – 5 position, a phenyl group is present. For the alcohol we add the suffix "ol".
So, the IUPAC name of CH3−CH(C6H5)−CH2−CH(OH)+CH2C−H3 is 5 – Phenyl – 3 – hexanol.
Therefore, the correct answer is option (C).
Note: We should find and the name the longest continuous carbon chain and we should identify and name groups attached to the chain, then we should number the chain consecutively, starting at the end nearest a substituent group and at last we should designate the position of each substituent group by an appropriate number and name. Also, the name of the compound should be written out with the substituents in the alphabetical order which is followed by the base name.
