Question
Question: The IUPAC name of the compound \(C{{H}_{3}}CH=CHC\equiv CH\) is: [A] pent-4-yn-2-ene [B] pent-3-...
The IUPAC name of the compound CH3CH=CHC≡CH is:
[A] pent-4-yn-2-ene
[B] pent-3-en-1-yne
[C] pent-2-en-4-yne
[D] pent-I-yn-3-ene
Solution
IUPAC is a worldwide accepted naming system. To name this compound, firstly identify the functional groups present if any. Then count the number of carbon atoms here in the parent chain and it will be the prefix. Then identify the position of alkene and alkyne and including it in the prefix will give you the IUPAC name of the given structure.
Complete step by step answer:
We know that IUPAC nomenclature is a method of naming chemical compounds as recommended by the International Union of Pure and Applied Chemistry. There are certain steps that we have to follow while writing the IUPAC name of any compound.
Firstly, let us write the structure and number the carbon atoms in it.
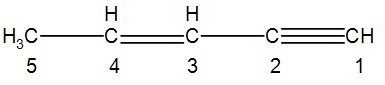
We start numbering the carbon atoms from the triple bond end.
We can see that we have no functional group here so we can move onto the next step which is counting the number of carbon atoms in the parent chain.
We can see that there is no branching in the given compound and the number of carbon atoms in the parent chain is 5.
Therefore, in the IUPAC name we will have the prefix –pent.
Now we can see that we have a double bond at the third carbon and a triple bond at the first carbon.
For the double bond we add the suffix –ene and for the triple bond we add the suffix –yne.
Here, we have both double and triple bonds in the same chain so we will add the carbon numbers before the designated suffix i.e. 3-ene and 1-yne.
While writing the IUPAC name, priority is given to the double bond so we have to write it as -3-ene-1-yne and not 1-yne-3-ene.
So, if we add the prefix –pent to this we will get the IUPAC name as pent-3-en-1-yne.
So, the correct answer is “Option B”.
Note: To write the IUPAC name of any compound, we should remember the basic principle that while naming any compound in its IUPAC name, we generally start with the number of carbons in the parent chain. In the suffix, we have the name of the functional group attached to the parent chain. The other groups which are present are the substituents.
