Question
Question: The IUPAC name of the compound above is: 
(A) Ethoxypropane
(B) 1,1− dimethyl ether
(C) 2− ethoxy isopropane
(D) 2− ethoxypropane
Solution
The IUPAC stands for the International Union of Pure and Applied Chemistry. The IUPAC name is given to the compound based on rules for the nomenclature of compounds. The basic step to give the IUPAC name of the compound is to decide the parent chain.
Complete step by step answer:
We can easily predict or figure out the IUPAC name of the given compound if we follow the procedure of nomenclature of compounds step by step. So, we know that the basic step for the nomenclature of the compound is to figure out the parent chain. This rule is also known as the chain rule. So the parent chain contains the maximum possible number of carbon atoms in a chain. So here the parent chain with the maximum possible number of carbon atoms is given below.
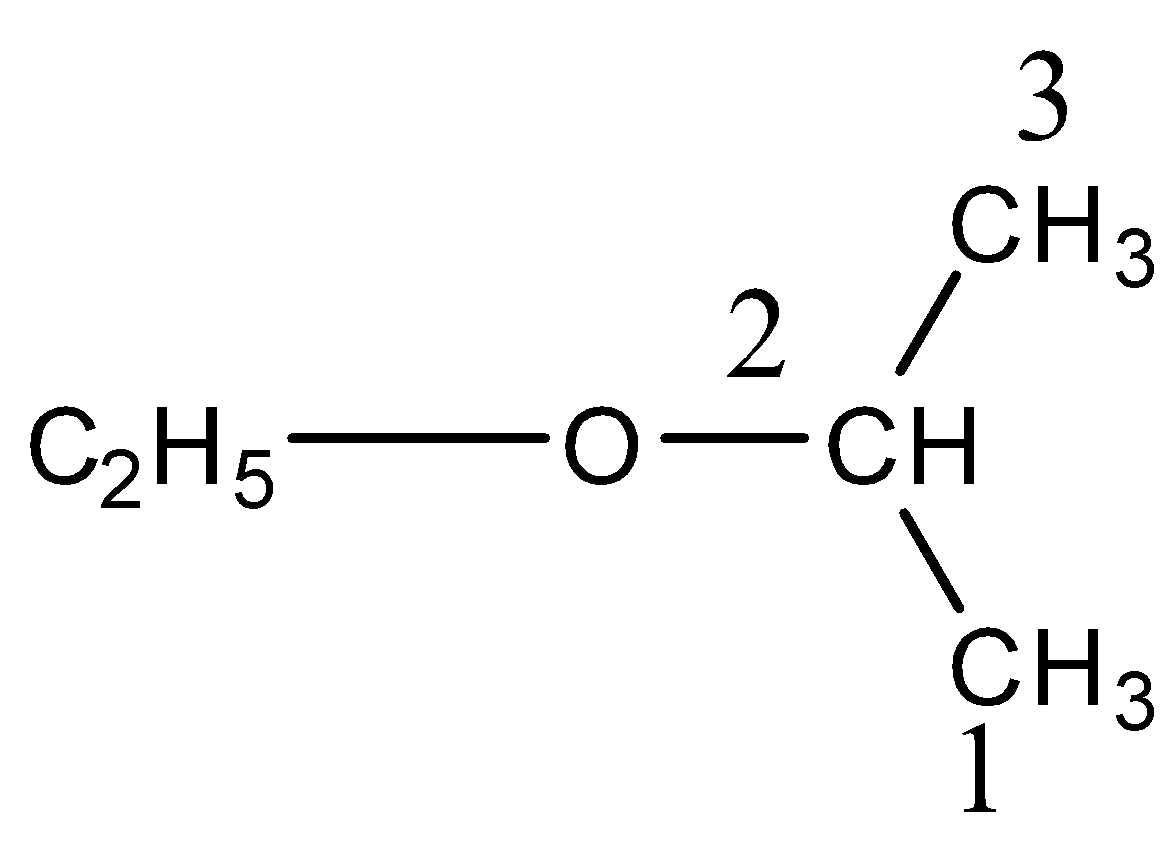
From the structure and the numbering of carbon atoms, we can easily observe that the maximum possible number of carbon atoms in a chain is three. So, it will be considered as the parent chain. We can also observe that there is an ether present on the second carbon atom. So now in the IUPAC name, the parent name will be propane due to 3 the carbon atom chain. Hence, the IUPAC name of the compound is 2− ethoxypropane.
Therefore, the correct option is (D).
Note:
If the two chains are of equal lengths are possible then one with more number side chains is considered as the parent chain. The common name of the compound 2− ethoxypropane is Ethyl isopropyl ether. The Ethoxy group is studied separately as the functional group.
