Question
Question: The IUPAC name of \({\text{C}}{{\text{H}}_{\text{3}}}{\text{CH(C}}{{\text{H}}_{\text{2}}}{\text{C}}{...
The IUPAC name of CH3CH(CH2CH3)CH2CH3 is
1.) 1, 1-methylethyl propane
2.) 2-ethylbutane
3.) 1-methyl-1-ethyl propane
4.) 3-methylpentane
Solution
The structure will be well understood when written in bond structural form. The carbon skeleton may or may not be a straight chain.
Complete step by step answer:
IUPAC is an abbreviation for International Union of Pure and Applied Chemistry.
IUPAC nomenclature is used for naming organic compound molecules. This method is used so that from the name we can construct an unmistakable structural formula. Inorganic chemistry also has an IUPAC nomenclature. Some compounds have long IUPAC names, in that case trivial names are used. While in some cases IUPAC names are simpler than trivial names.
Following the rules of IUPAC, we will see how to assign the nomenclature step by step.
Step 1:- The longest chain rule: The parent chain of the compound is the longest chain. It may be a straight chain or chain of other shapes. Following this rule, the parent chain of the compound given is as follows:
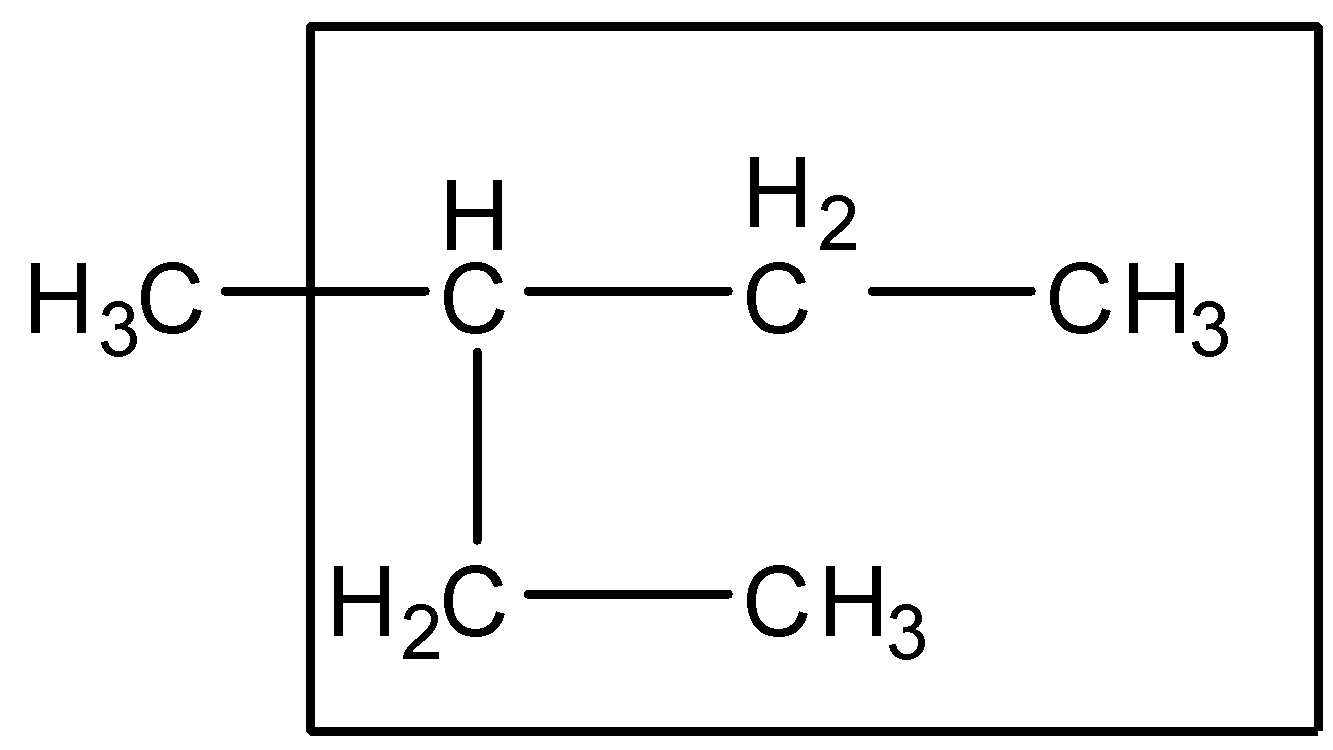
So accordingly we can see that the longest chain consists of 5 carbon atoms. Therefore the parent chain is Pentane.
Step 2:- The lowest set of locants: The carbons which belong to the longest carbon chain should be numbered. The numbers should begin from the end in which the lowest number is given to the carbon atom attached to the substituents.
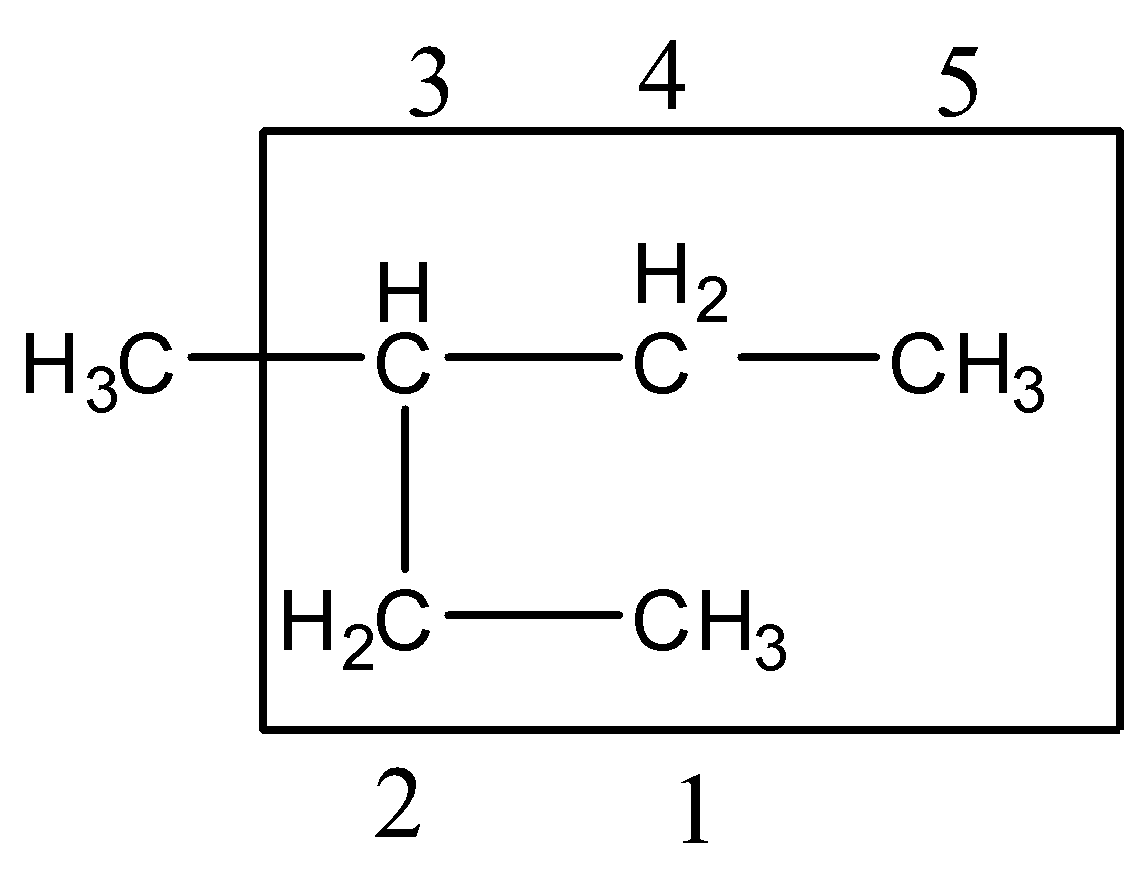
Since the substituent is attached to the center carbon atom, we can number from any one end. The substituent gets number 3.
Step 3:- Multiple instances of the same substituents: prefixes are used which indicate the total number of same substituents. Prefixes are di, tri, etc.
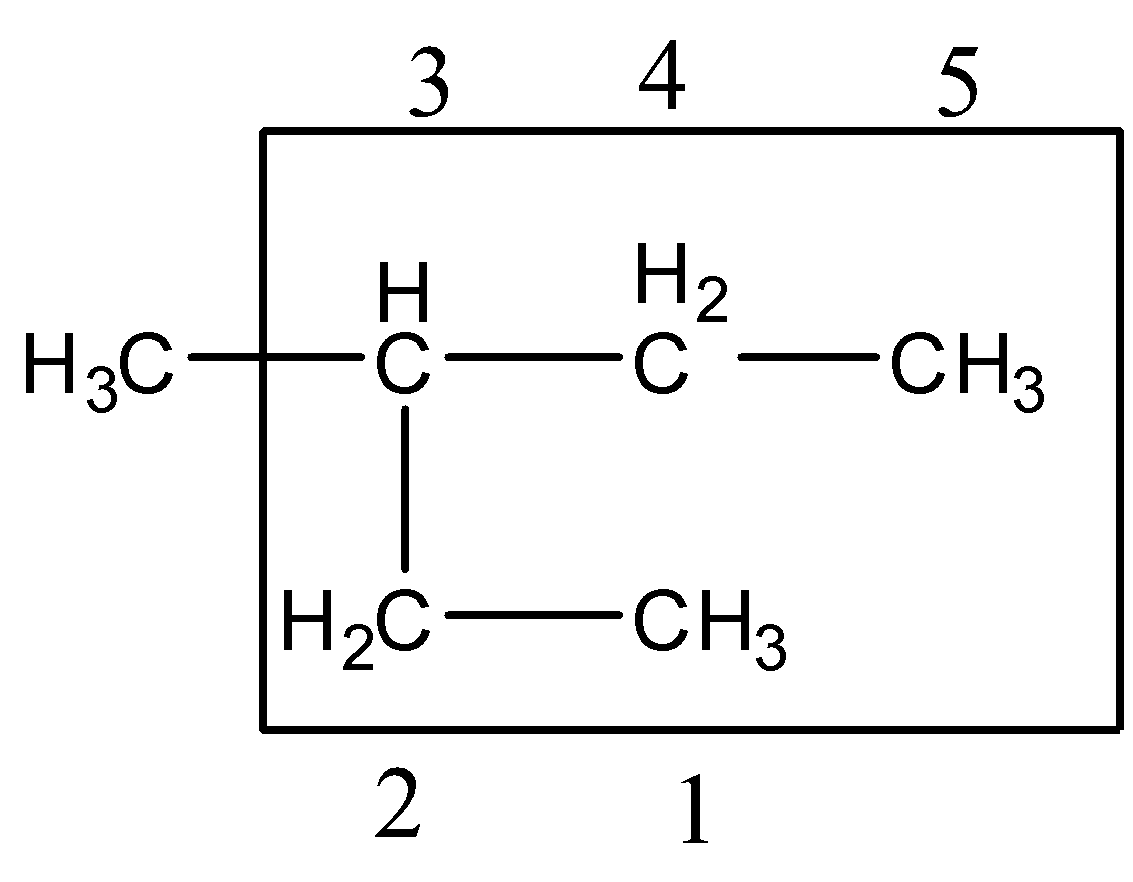
There is only one substitute attached so no need for any prefix.
The format of the IUPAC nomenclature is as follows: Locant + prefix + root + locanto +suffix.
As there is no functional group attached there is no need for the last two parts.
So the IUPAC name for CH3CH(CH2CH3)CH2CH3 will be: 3-Methylpentane.
So, the correct answer is “Option D”.
Note: If there are different substituents attached to the carbon chain, they are arranged then in the alphabetical order of names. If there is any functional group attached to the carbon chain then they are written in the form of suffix.
