Question
Question: The IUPAC name of \({{\text{C}}_{6}}{{\text{H}}_{4}}\text{COCl}\)is: A. Benzoyl chloride B. Benz...
The IUPAC name of C6H4COClis:
A. Benzoyl chloride
B. Benzene chloro ketone
C. Benzene carbonyl chloride
D. Chlorophenyl ketone
Solution
Benzene is a six-carbon containing compound. The naming of carbon is done according to the amount of it present in the molecule. Ketone is a -CO functional group whereas while doing the naming of halogens, they are commonly named as chloride, fluoride, bromide, etc.
Complete step by step solution:
- In the given question we have to explain the IUPAC or International Union of Applied and Pure Chemistry of the given compound.
- So, the structure of the compound is:
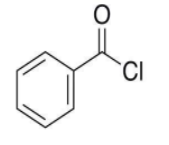
- Here, we can see that a chlorine group is attached at the benzoyl group i.e. C6H5CO-.
- We know that the IUPAC name of chlorine is chloride.
- So, the IUPAC name of the above compound is Benzoyl chloride.
- In benzene chloro ketone, the chlorine is attached to the other carbon other than carbonyl carbon.
- So, this option (b) is not correct because the benzoyl group is C6H5CO-.
- In option (c), the carbonyl group is mentioned whose formula is C=O, the particular group is present in the compound, but it cannot be considered as a prior group,
- So, option (c) is also incorrect.
- A phenyl group is similar to the benzene except it has one hydrogen atom less than the benzene.
- In the phenyl group, one hydrogen is absent and other elements can attach to its place.
- So, option (d) is also incorrect.
Therefore, option (a) is the correct answer.
Note: One may get confused with the benzene, benzyl and benzyl group. There is a difference between these three structures. The formula of benzene, benzyl and benzoyl is C6H5, C6H5CH2−,C6H5CO respectively.
