Question
Question: The IUPAC name of tertiary butyl chloride is: 
(a) 1-phenylethane
(b) ethyl phenyl ether
(c) m-butyl hydroxybenzene
(d) m-butylphenol
Solution
While writing the IUPAC name of any compound, we will first identify the functional group and to which the functional group is attached, it is taken as the main substituent and the others are taken to be as the side substituents. Now with the help of this you can easily write the name of the compound.
Complete step-by-step answer: The IUPAC stands for the International Union of Pure and Applied Chemistry and it is the method that is used for the naming of the organic or inorganic compounds and the compounds are known by their IUPAC naming all over.
The given compound consists of the benzene ring with OH group attached at the ortho position and the butyl group is present at the meta position.
Since, the functional group i.e. OH group is attached to the benzene ring, so, the benzene ring along with the OH group is considered as the main ring and the butyl group attached to it is considered as the side substituent.
While writing the IUPAC name, the name of the substituent will be written first followed by the name of the main ring.
The butyl group is attached to the benzene ring through the meta position , so while writing the IUPAC name of substituent, its position of attachment to the ring is also specified.
So, now the IUPAC name of the compound having the structural formula as;
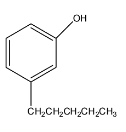
is; m-butylphenol .
Hence, option(d) is correct.
Note: While naming the aromatic compound, always keep in mind that if the functional group is attached to the compound , then it is the main ring and the attached carbon chain is the substituent and if there is no functional group, then the carbon chain acts as the main chain and benzene ring acts as the substituent.
