Question
Question: The IUPAC name of tertiary butyl bromide is _____....
The IUPAC name of tertiary butyl bromide is _____.
Solution
For IUPAC nomenclature of branched chain alkanes, the longest continuous carbon chain is taken as the parent chain and then the parent chain is numbered in such a way that the numbering starts from that end which is nearest to the substituent.
In IUPAC nomenclature of organic compounds, a tertiary group is used as a prefix before the name of the alkyl group which indicates that the hydrogen atom removed was a tertiary hydrogen atom.
Complete step by step answer:
When a functional group is present, then the longest continuous chain containing the functional group is considered as the parent chain and numbering is done such that the functional group gets the lowest position. But, the halo groups and the nitro groups are always taken as substituents.
A tertiary butyl group has the following structure.
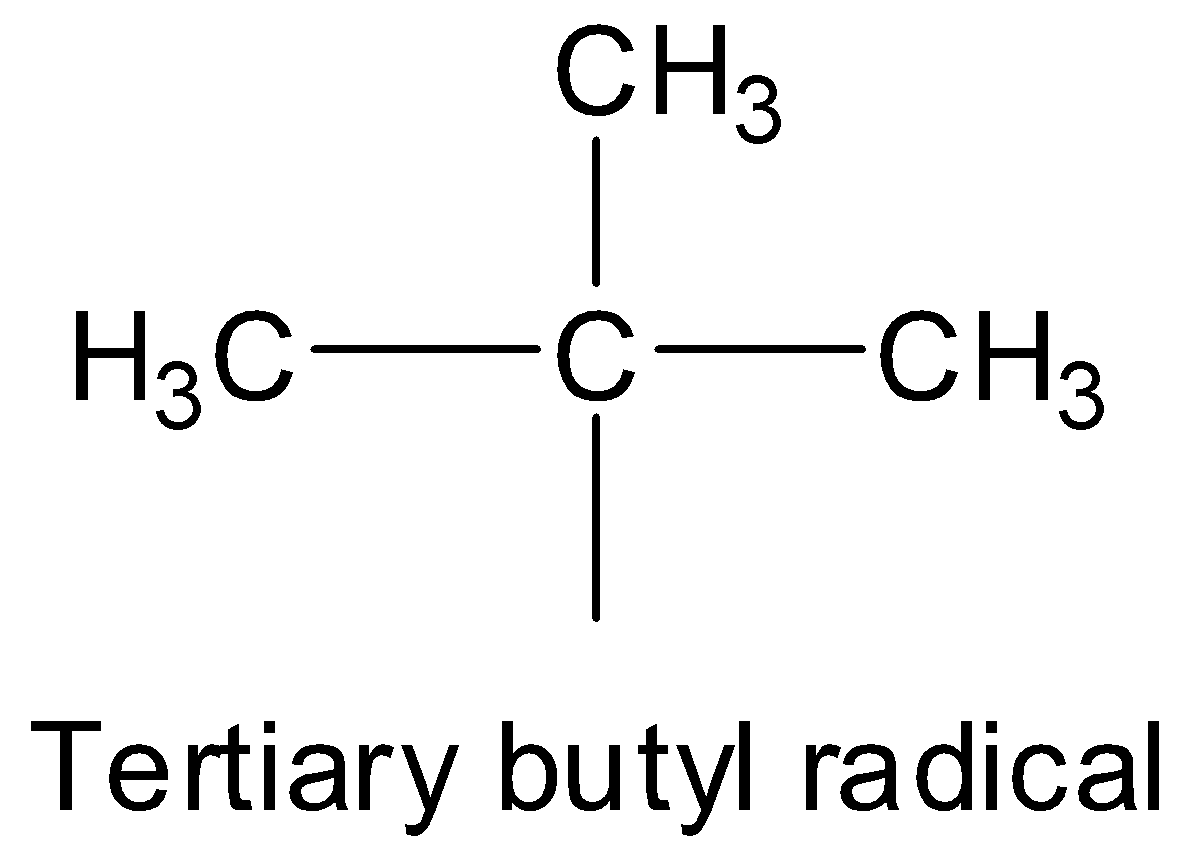
The name of the compound is tertiary butyl bromide, which means there is a bromine atom attached to the tertiary carbon atom of the tertiary butyl group. So, the complete structure of tertiary butyl bromide will be:
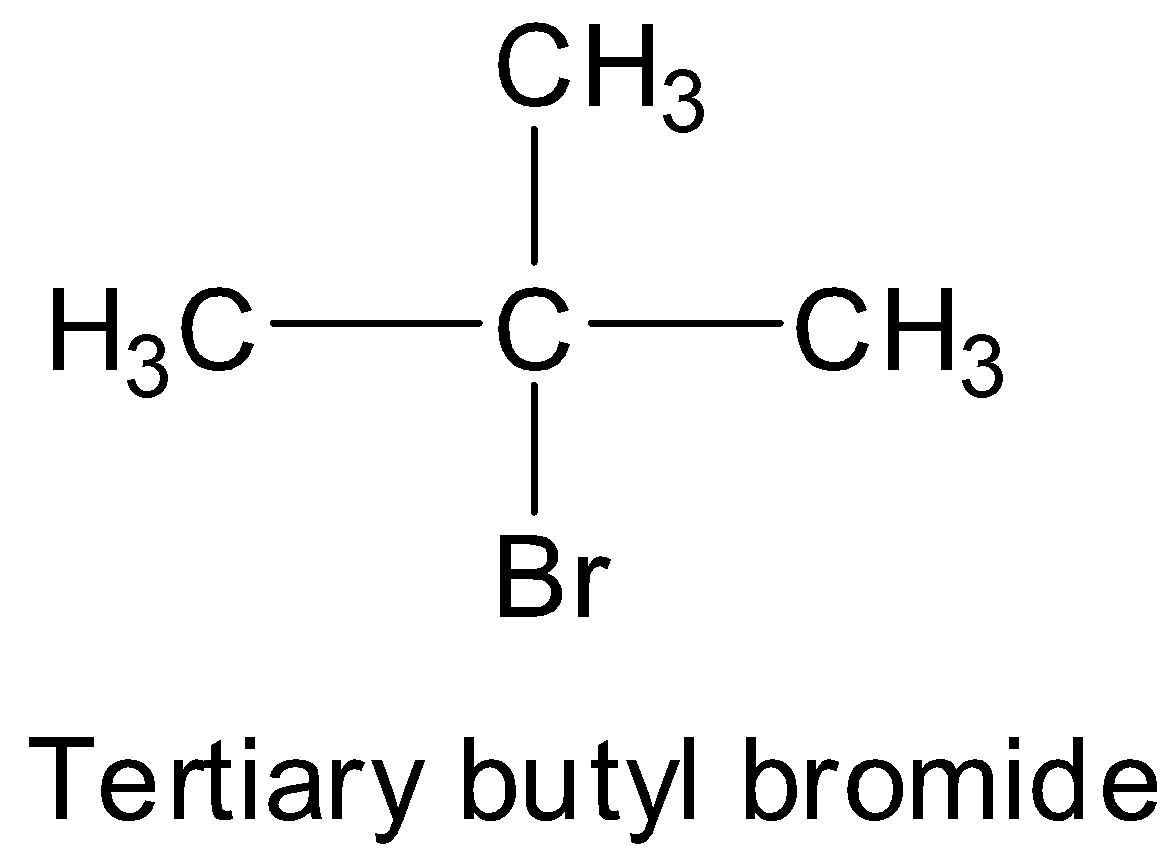
Here, the longest continuous carbon chain consists of three carbon atoms. And three carbons indicate propane. So, the parent chain is propane.
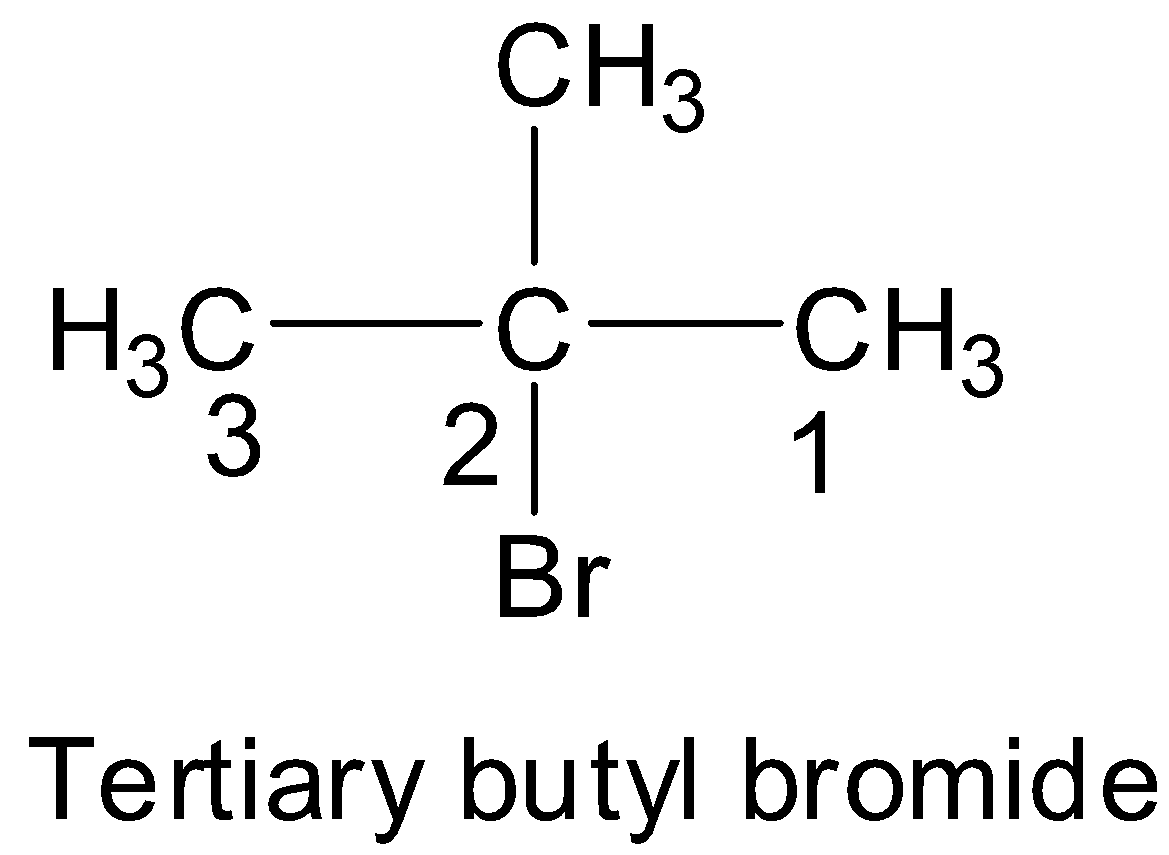
Here, the substituents are a methyl group and a bromine group and so numbering is done in such a way that it starts from the end which is nearest to these groups. In this case, numbering from both the ends of the chain is equivalent.
Now, we can see that both the methyl and bromo substituents are present at 2- position. According to the IUPAC rules, when there are two or more different substituents present, the groups are arranged in alphabetical order. Hence, the IUPAC name of tertiary butyl bromide is 2- bromo- 2- methylpropane.
Note:
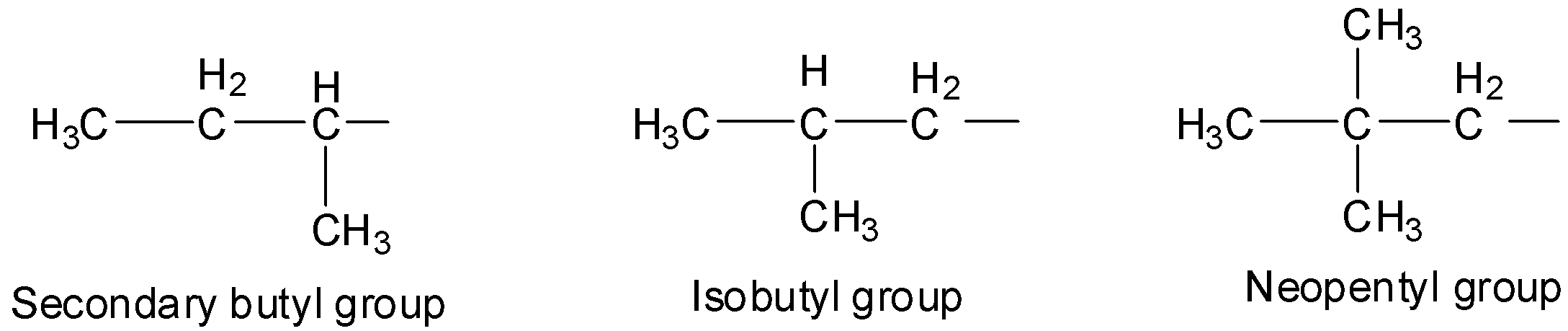
A secondary group is used as a prefix before the name of the alkyl group which indicates that the hydrogen atom removed was a secondary hydrogen atom. The prefix iso is used when only one methyl group is attached to the second last carbon of the continuous chain. The prefix neo is used in such cases when two methyl groups are attached to the second last carbon of the continuous chain.