Question
Question: The IUPAC name of 
is?
Solution
CHO attached to a compound indicates the presence of the functional group called Aldehyde. They are prepared from hydrocarbons and by oxidation of primary and secondary alcohol. Usually aldehydes are liquid or solid at room temperature. Only methanol is a gas at room temperature and ethanal is volatile liquid.
Complete step by step answer:
There are various functional groups attached to chemical compounds; one such functional group is the Aldehyde group which is present due to the presence of CHO group in the compound. This functional group plays a very important role in biochemical processes of life, it has pleasant fragrance and is for adding flavours to food.
The compound with this functional group has –al at the end of their names.
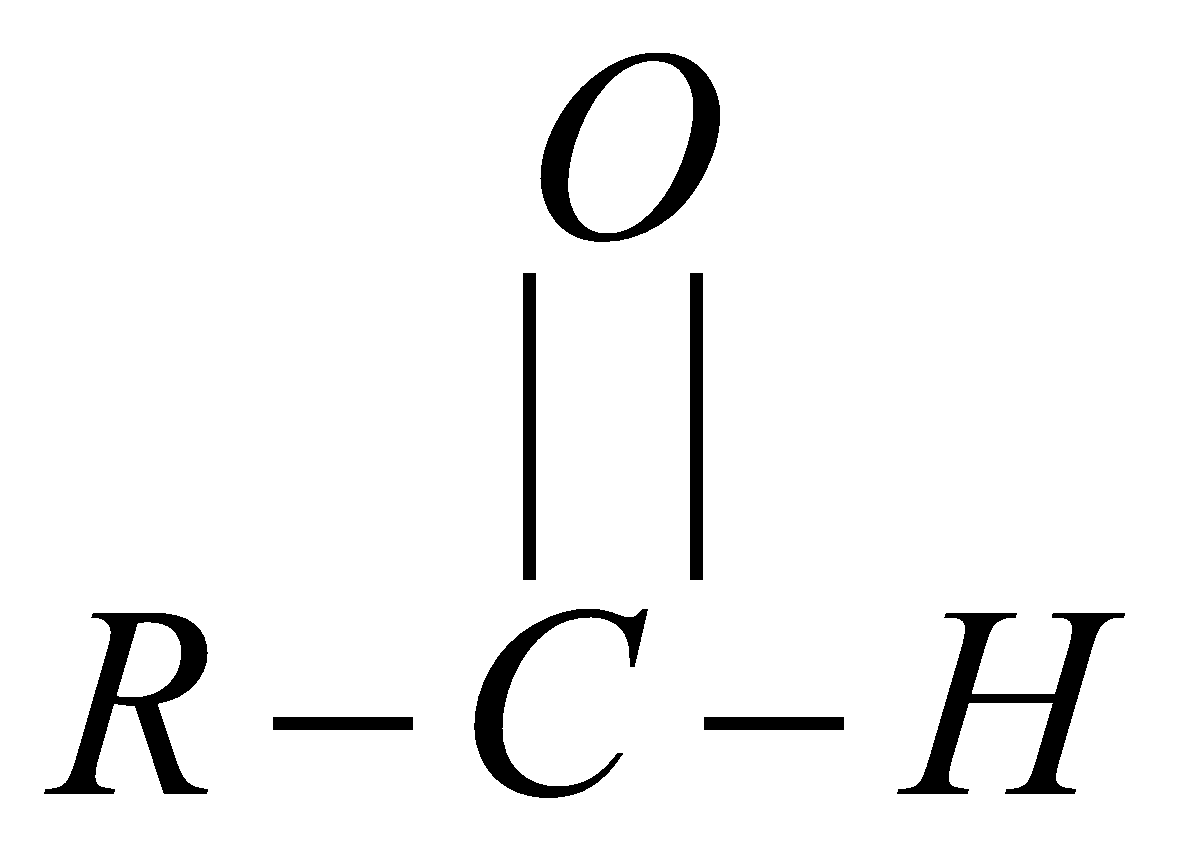
This is how the CHO group is attached to any compound.
The lower members of this functional group like methanol, ethanol dissolve in water easily in all proportions as they form hydrogen bonds with water which is a strong bond. But the solubility of aldehydes decreases with the increasing length of the compound. The boiling point of Aldehydes is higher than other hydrocarbons because of the weak intermolecular forces due to dipole-dipole interactions.
Now to write the IUPAC name of the given compound we need to remember certain points like looking for the longest carbon chain, identifying the functional groups attached and naming the single, double and triple bonds according to their position and the branches of each atom.
Thus the IUPAC name of the above compound is: 3-ethenyl-2-hept-2-en-6-ynal. CHO is the functional group in the given compound.
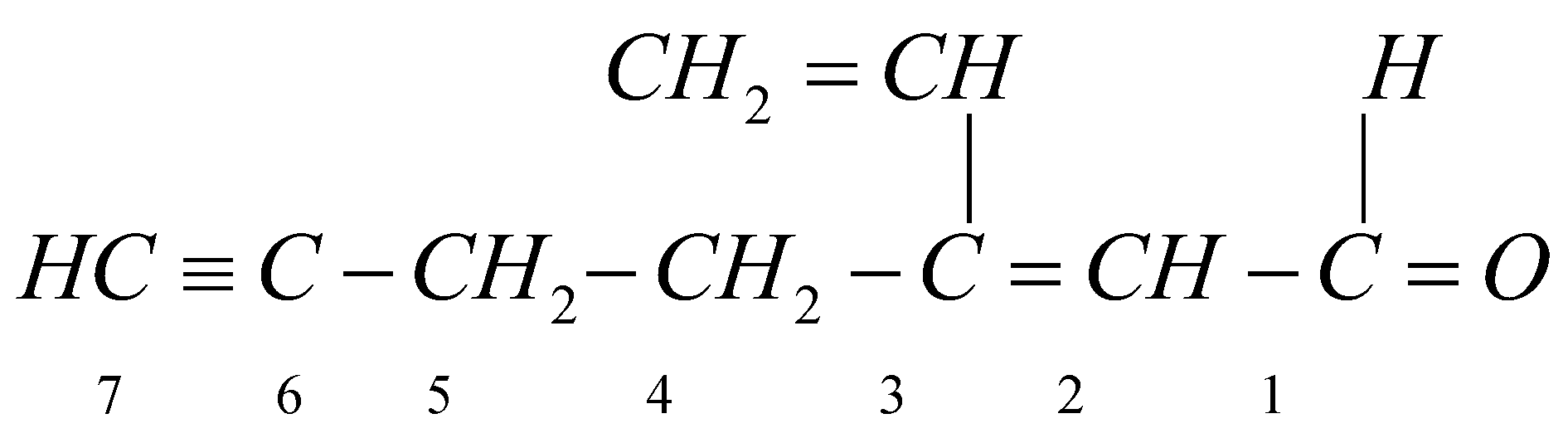
Thus we number the carbon atoms starting from the carbon atom of the functional group.
Note: There are certain tests which enable us to distinguish between an Aldehyde and a ketone one of which is Tollen’s test in which if an aldehyde is heated with a freshly prepared ammoniacal silver nitrate solution then, a silver mirror is produced because of the formation of a silver metal.
