Question
Question: The IUPAC name of 
A. 1 methyl pent-1-ene
B. 2-methyl pent-4-ene
C. 4-methyl pent-1-ene
D. 2-methyl pent-1-ene
Solution
Hint According to IUPAC (International Union of Pure and Applied Chemistry), whenever we are going to write the IUPAC name of a compound, we have to give numbering first to functional groups or highly substituted carbon. Means lower numbering should be a functional group or highly substituted carbon present in the molecule or compound.
Complete step by step answer:
- In the question it is asked to write the IUPAC name of the given compound.
- The given structure of the compound is as follows.
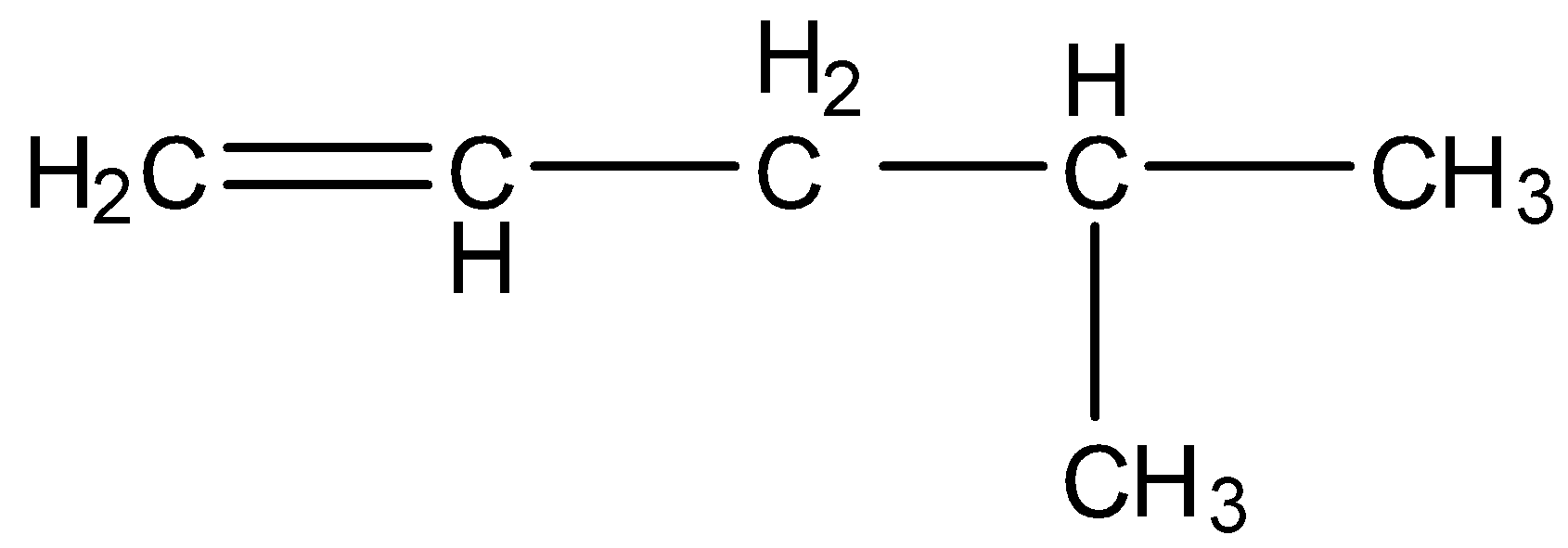
- We have to give numbering to all the carbons to the given compound and the least number should be given to the functional group which is present in the compound.
- The given compound has an alkene as a functional group and the numbering to the given compound is as follows.
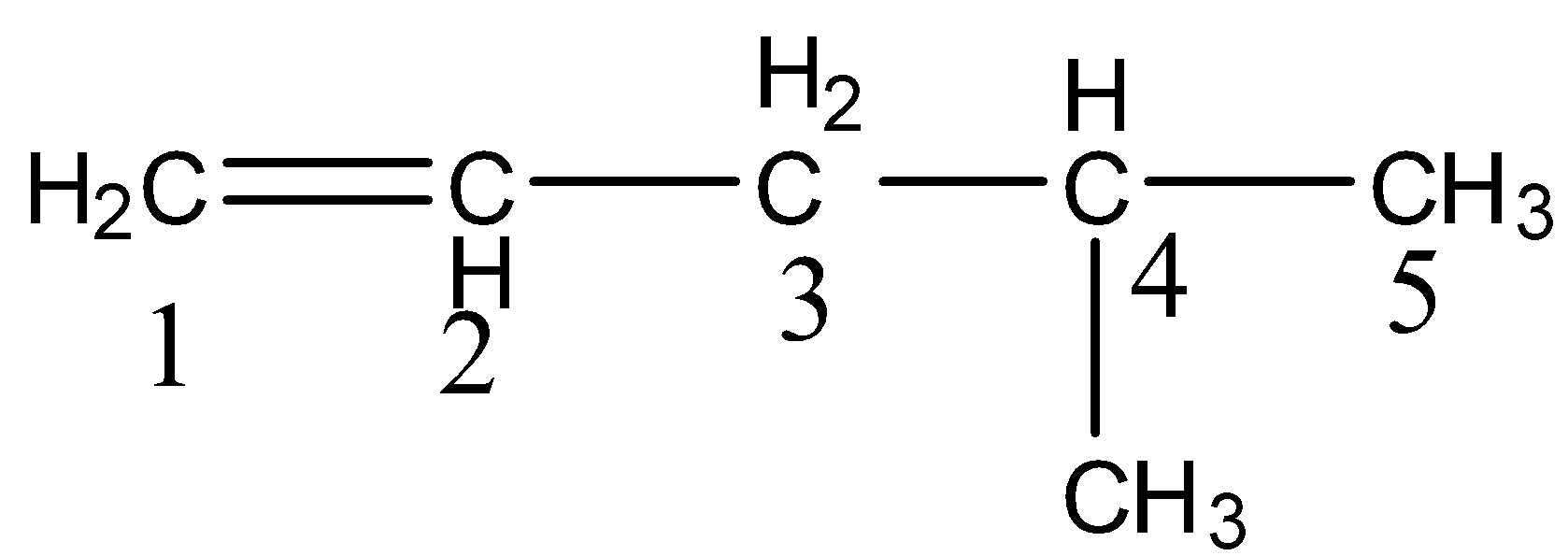
- There are five carbons present in the longest chain then the name pentane is supposed to be written in the IUPAC name.
- There is a methyl group attached to carbon-4 as a substituent then the name – 4-methyl should come in IUPAC name.
- There is an alkene functional group at carbon-1 then in the IUPAC name ‘1-ene’ should come.
- Totally the name of the given compound will be 4-methyl pent-1-ene.
- So, the correct option is C.
Note: If we give numbering in a reverse direction means high number to alkene and lower number to the remaining carbons then the IUPAC name of the compound will be 2-methyl pent-4-ene. But it is wrong because lower or least numbers should be given to functional groups.
