Question
Question: The IUPAC name of \[C{H_3} - C\left( {C{H_3}} \right)\left( {OH} \right)C{H_2} - CH{\left( {CH{}_3} ...
The IUPAC name of CH3−C(CH3)(OH)CH2−CH(CH3)2 is
A. 2,4-dimethylpenta-2-ol
B. 2,4-dimethylpenta-4-ol
C. 2,2-dimethylbutane
D. 2,4,4-trimethylbutan-2-ol
Solution
The carbon which is attached to the preferred functional group will begin the numbering of carbon atoms. The lower the number bearing the functional group is lowered.
Complete step by step answer:
There are certain rules for naming the compounds following IUPAC nomenclature. At first the functional group has to be identified. In this case it is an alcohol specifically a tertiary alcohol. Also the given hydrocarbon is a branched chain hydrocarbon.
For naming branched chain numbering is done so that the maximum number of carbon atoms is numbered leading to a longest possible chain. Thus the maximum of carbon atoms is five for the given compound. So it is a pentane alkane chain.
The alcohol is named by replacing the suffix –ane with -anol. The position of the hydroxyl group written on the parent chain is indicated by placing the number in front of the base alkane name.
The hydroxyl group takes precedence over other alkyl or halogen substituents attached to the parent chain. If double bonds and hydroxyl groups are present the –en suffix is followed by ol.
Thus the numbering of the carbon atom is done as follows:
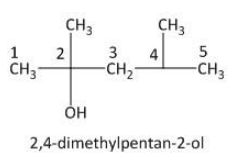
From the above picture the hydroxyl group is attached to the C2 carbon atom. Two methyl groups are attached to the parent hydrocarbon chain, one at C2 and one atC4. Thus the IUPAC name of the given compound is 2,4-dimethylpenta-2-ol.
So, the correct answer is “Option A”.
Note:
A molecule’s longest chain of carbons connected by single bonds is considered as the base for naming compounds by IUPAC nomenclature. The chain can be a continuous chain or a ring.
