Question
Question: The IUPAC name of \( C{H_3}C{H_2}COC{H_2}C{H_3} \) is (A) 2-pentanone (B) 3-pentanone (C) Diet...
The IUPAC name of CH3CH2COCH2CH3 is
(A) 2-pentanone
(B) 3-pentanone
(C) Diethyl ketone
(D) All of the above
Solution
IUPAC nomenclature is a method of naming the organic compounds recommended by the International Union of Pure and Applied Chemistry. IUPAC names are usually a long and tedious method of naming the compounds and chemists do not always prefer IUPAC naming. Trivial names or common names are used more often.
Complete answer:
The question given above is to find out the IUPAC name of the compound.
IUPAC nomenclature has some rules that one should follow to name any compound. The rules given are as follows:
-At first, the longest continuous carbon chain is chosen as a parent name of the compound
-Next, all the carbon atoms chosen as parent chains are numbered. Numbering will start from terminal carbon. It should be in such a way that the substituent gets the lowest number.
-Then name each of the substituents and indicate each of them by number.
Finally, combine the name and the substituent and the parent chain position and write it as one word.
After knowing the rules apply the rules to name the above compound.
The given compound is CH3CH2COCH2CH3
Here the longest continuous chain has 5 carbons and so the longest chain is pentane as no double or triple bonds are present. Now the numbering of the chain will be as follows:
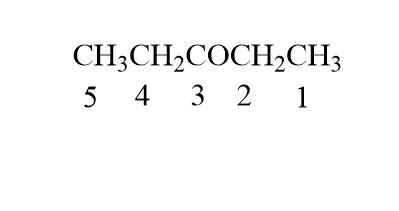
As we can see above the 3rd carbon has functional group ketone hence the parent chain will end with the suffix –one.
Therefore the IUPAC name of the compound is 3−pentanone.
So the correct option is B.
Note:
The name 3−pentanone can also be written as pentane−3−one . If a double bond or triple bond is present, the compound’s name will end with ‘ene’ for double bond and ‘yne’ for a triple bond. The parent chain in some cases is a ring also. The groups’ alcohol, -COOH, ketone, etc. always are present in the suffix in IUPAC naming.
