Question
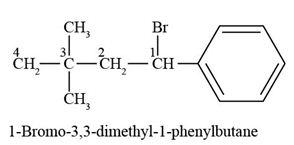
Question: The IUPAC Name of \[{{(C{{H}_{3}})}_{3}}CC{{H}_{2}}CH\left( Br \right){{C}_{6}}{{H}_{5}}\] is...
The IUPAC Name of (CH3)3CCH2CH(Br)C6H5 is
Solution
The International Union for the Standardization of Nomenclature in Chemistry (IUPAC) is most recognized for its work standardizing nomenclature in chemistry, although it has publications in a wide range of research areas, including chemistry, biology, and physics. Standardizing nucleotide base sequence code names, producing publications for environmental scientists, chemists, and physicists, and enhancing scientific education are some of the major things IUPAC has done in these disciplines.
Complete answer:
The benzene ring is identified by adding a phenyl prefix to the alkane name for substituted benzene rings with more than six carbons.
The alkyl chain is added as a prefix to substituted benzene rings that have less than six carbons, and the termination is altered to -yl.
The ring atoms are numbered for benzene rings with numerous substituents to reduce the numbering of substituent groups; alternatively, ortho/meta/para nomenclature can be employed for disubstituted rings.
When a benzene ring has just one substituent and the substituent has six or less carbons, the substituent is added as a prefix to benzene. The alkyl groups are called using the alkane series convention, which ends in -yl: methyl (one carbon), ethyl (two carbons), propyl (three carbons), and so on.
In the case of compounds having more than one substituent group present in the benzene ring, you must use Greek numeral prefixes such as di, tri, and tetra to identify comparable substituent groups.
If various substituent groups are present in aromatic compounds, the base's substituent should be assigned to the first position. Furthermore, the rest of the compound's numbering direction is set so that the following substituent will have the lowest numbering position. Furthermore, while naming the substituent, we must utilize alphabetical order.
Hence the IUPAC name of (CH3)3CCH2CH(Br)C6H5 is 1-Bromo-3,3-dimethyl-1-phenylbutane
Note:
In the case of aromatic compounds with several substituents, prefixes such as ortho, meta, and para must be used to denote relative positions such as 1,2-; 1,3-; 1,4-. We can, for example, rephrase 1,2 dibromobenzene as o-dibromobenzene.
When an organic compound consists of an alkane with a functional group and an aromatic compound, the aromatic compound replaces the parent group as a substituent. When a benzene ring is linked with an alkane and a functional group, the aromatic group is referred to as phenyl (Ph-).
