Question
Question: The IUPAC name of below is:\({{(C{{H}_{3}})}_{2}}CH-CH=CH-CH=CH-CH({{C}_{2}}{{H}_{5}})-C{{H}_{3}}\) ...
The IUPAC name of below is:(CH3)2CH−CH=CH−CH=CH−CH(C2H5)−CH3
The IUPAC name of this is:
(A) 2,7-dimethyl-3,5-nonadiene
(B) 2,7-dimethyl-2-methylbutadiene
(C) 2-methyl-7-ethyl-3,5-octadiene
(D) 1,1-dimenthyl-6-ethyl-2,4-heptadiene
Solution
International union of pure and applied chemistry governs the nomenclature of organic chemical compounds. The name of a compound should be able to be used to depict and determine the structural formula of a chemical compound.
Complete answer:
Let us first redraw the compound for better clarity.
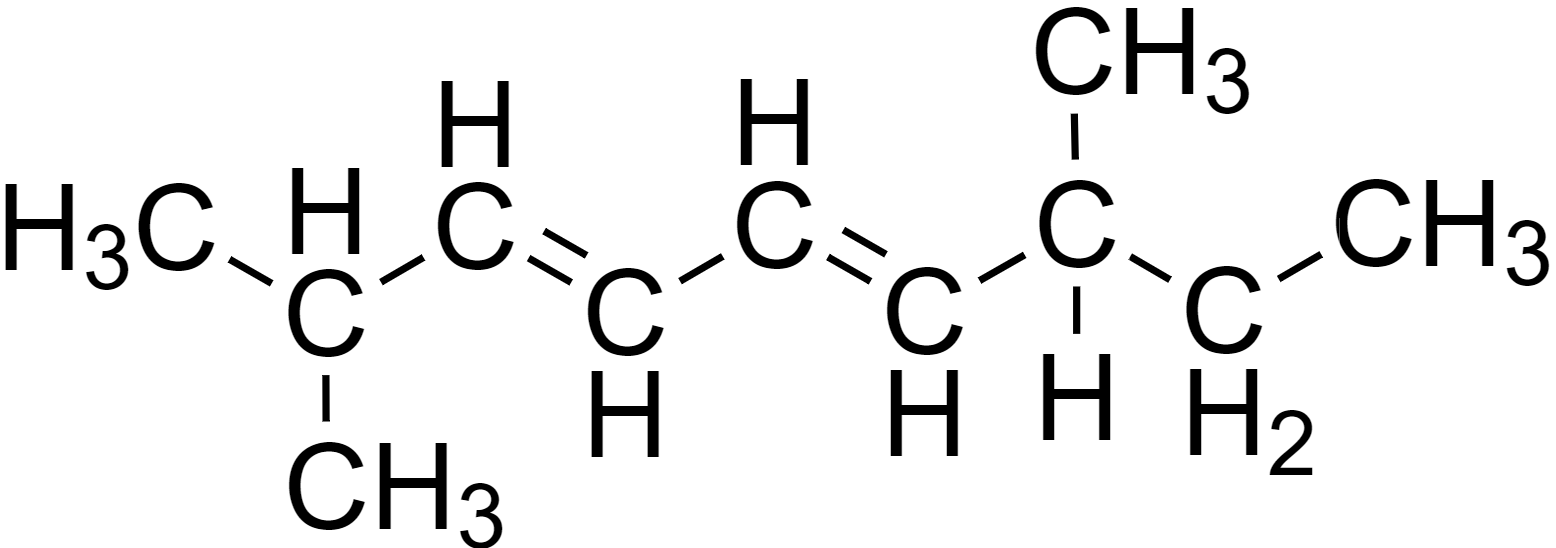
Now, the IUPAC nomenclature of the compound can be determined through the following steps.
1. Determine the parent hydrocarbon chain.
- The longest hydrocarbon chain is the parent hydrocarbon chain.
- In case there are two hydrocarbon chains with the same length, the chain with the most branching is the parent chain.
- The parent chain should have the maximum number of single bonds as well as double bonds.
Here the parent carbon chain is a 9 carbon chain (nona-) containing double bonds.
2. Identify the functional group.
Here there are no functional groups.
3. Identify the side chains.
Here there are two methyl chains.
4. The identification of unsaturated bonds.
Here there are two unsaturated double bonds.
5. Numbering the chain
The chain is numbered such that the chain has the lowest value. The unsaturated bonds and the side chains present must also have the least possible value.
Hence the chain will be numbered from left to right.
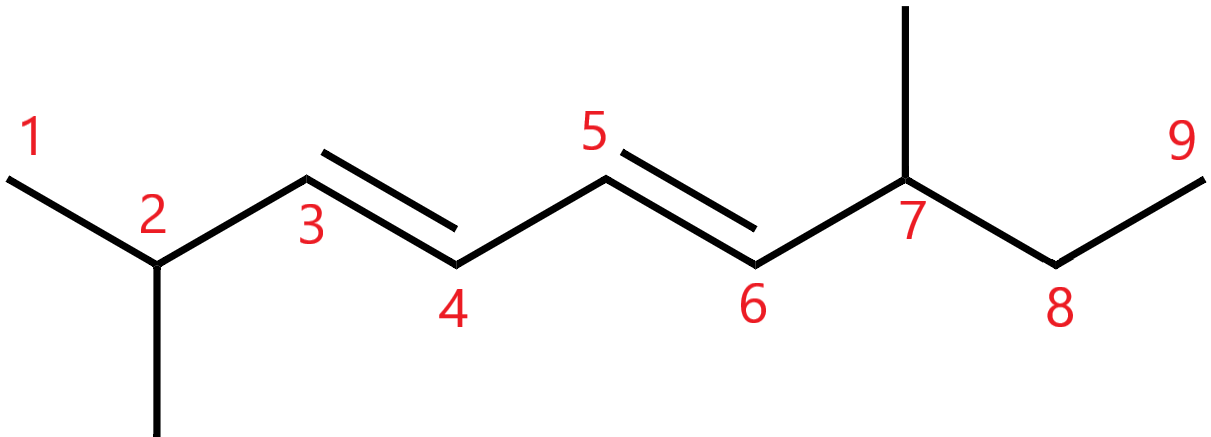
6. When there are multiple substituents of the same type, prefixes like di-, tri-, are added.
So, since there are two methyl groups and two double bonds, we will use dimethyl and diene.
7. Name the compound.
- The numbers are separated by commas and the sets of numbers are separated from words by hyphens (-).
So, the IUPAC name of the compound is option (A) 2,7-dimethyl-3,5-nonadiene.
Note:
It should be noted that while IUPAC nomenclature can help determine the structural formula of a compound, for complex molecules, they are tedious and long and hence are avoided in normal conversation. Simple and common names are used instead.
