Question
Question: The IUPAC name of aniline is: A. phenylamine B. aminobenzene C. benzylamine D. benzenamine...
The IUPAC name of aniline is:
A. phenylamine
B. aminobenzene
C. benzylamine
D. benzenamine
Solution
The phenyl ring is a cyclic ring which is viewed as a benzene ring having formula C6H6 with one hydrogen atom less (C6H6−H) where the hydrogen atom is replaced by other compounds. The molecular formula of the phenyl group is C6H5.
Complete step by step answer:
Aniline is a compound carrying two groups, one is a phenyl ring and the other is an amino group. The amino group is directly attached to the phenyl ring.
Preparation of aniline
When benzene is heated with concentrated sulphuric acid and concentrated nitric acid at 40∘C, nitrobenzene is formed. This reaction is known as nitration. On reacting nitrobenzene with concentrated hydrochloric acid and excess sodium hydroxide in presence of the tin catalyst, aniline is formed.
The reaction is shown below.
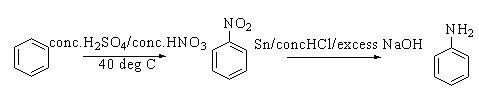
The chemical structure of aniline is shown below.
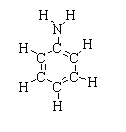
Aniline is commonly known as aminobenzene. The chemical formula of aniline is C6H5NH2or C6H7N. Aniline consists of six carbon atoms, seven hydrogen atoms, and one nitrogen atom.
As carbon is the chemical formula, aniline is considered as an organic compound. The phenyl is a hexagonal ring with an alternate double bond and the amino group consists of one nitrogen atom joined with two hydrogen atoms. The resulting compound is an aromatic amine.
The IUPAC name of aniline is phenylamine.
Therefore, the correct option is A.
Note:
The phenyl ring is closely connected with the benzene. The alternative name for aniline is given as benzene amine because of the structure. In benzylamine, one methyl group is added and thus the molecular formula of benzylamine is C6H5CH2NH2.
