Question
Question: The IUPAC name of an unsymmetrical ether with the molecular formula \({{\text{C}}_{\text{4}}}{{\text...
The IUPAC name of an unsymmetrical ether with the molecular formula C4H10 is:
(A) Ethoxy propane.
(B) Methoxy ethane.
(C) Ethoxy ethane.
(D) Methoxy propane.
Solution
We will first draw the structure of all the compounds given in the options and then compare them with the given molecular formula and also see whether it is unsymmetrical or not. Initially we count the number of carbon atoms in the carbon chain.
Complete step by step answer:
The naming of the ether is very simple; we see the substituents group on each side of the oxygen. The more complex substituent name becomes the root word and the less complex part is named as alkoxy.
Now we will see the structure of compounds of every option:
(A) Ethoxy propane 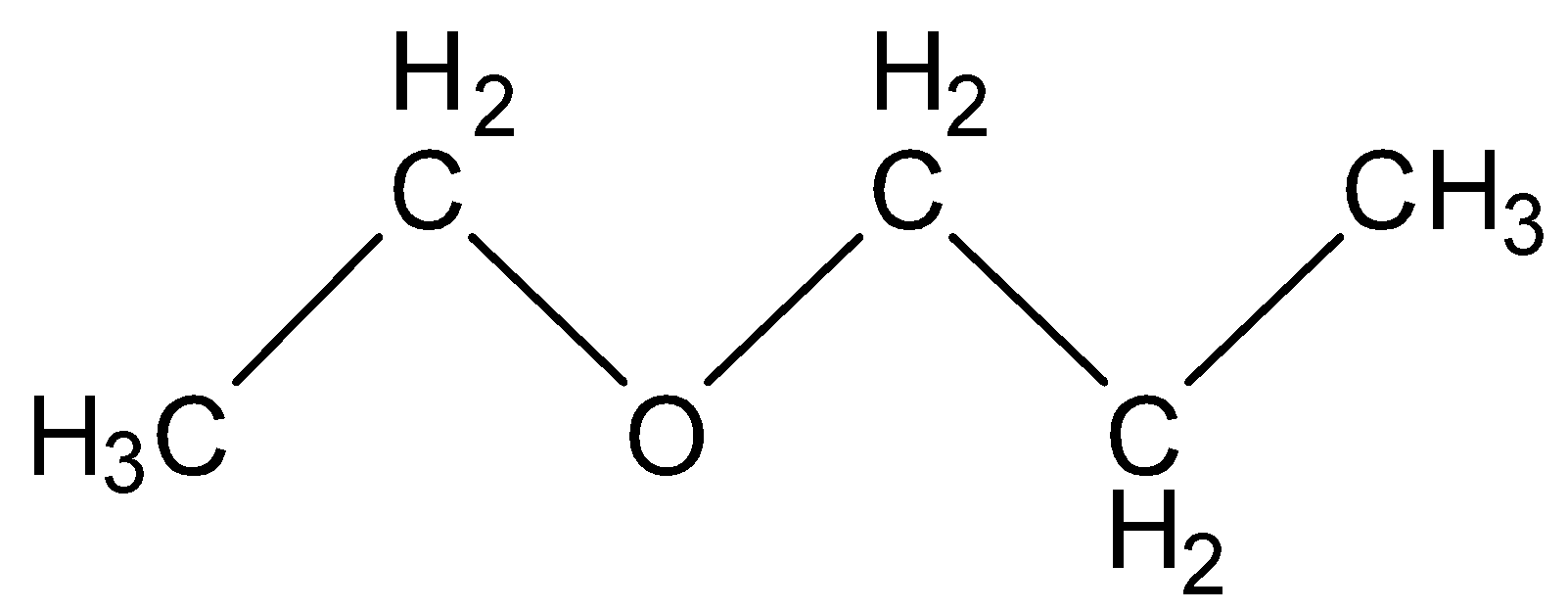
In this structure there are five carbons and twelve hydrogen present which is not equal to the molecular formula given in question above. So, it is an incorrect option.
(B) Methoxy ethane 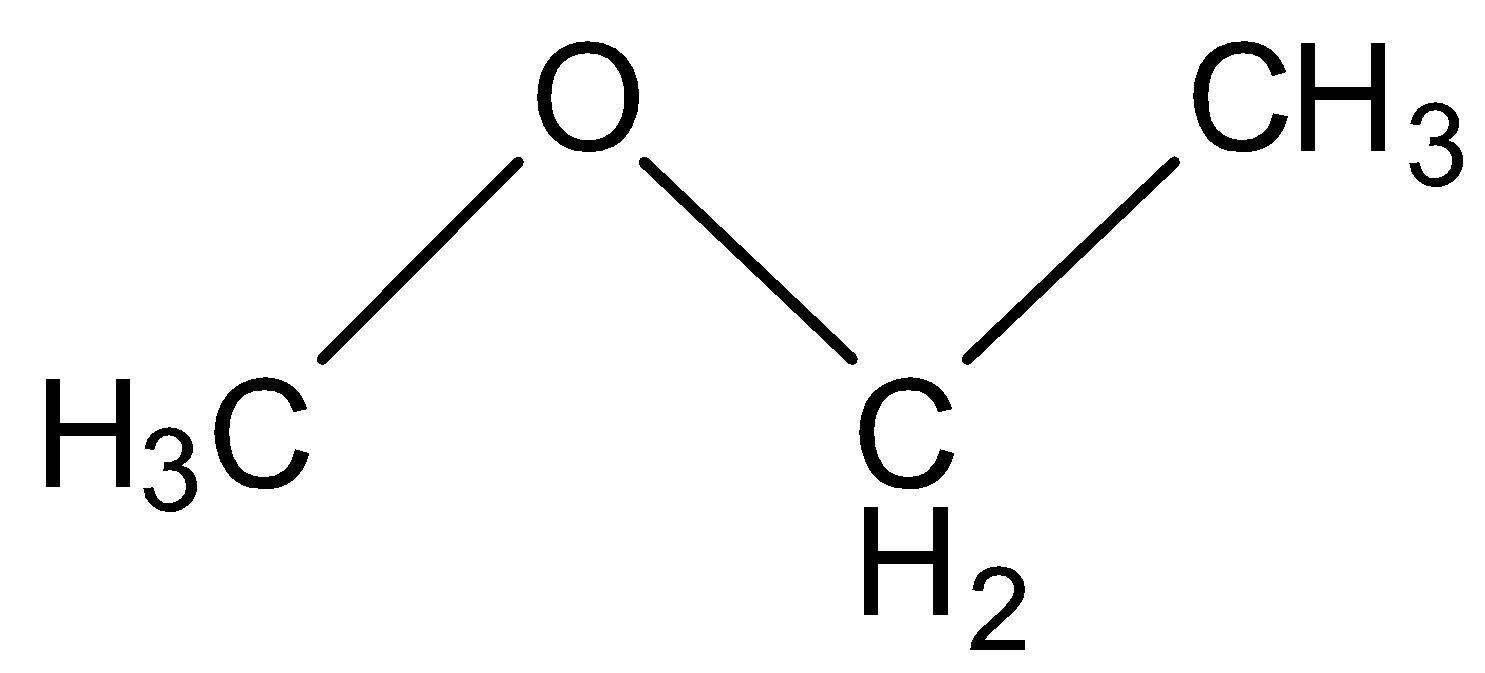
This structure contains three carbon and eight hydrogen which is again not equal to the molecular formula given in the question so this is also an incorrect option.
(C) Ethoxy ethane 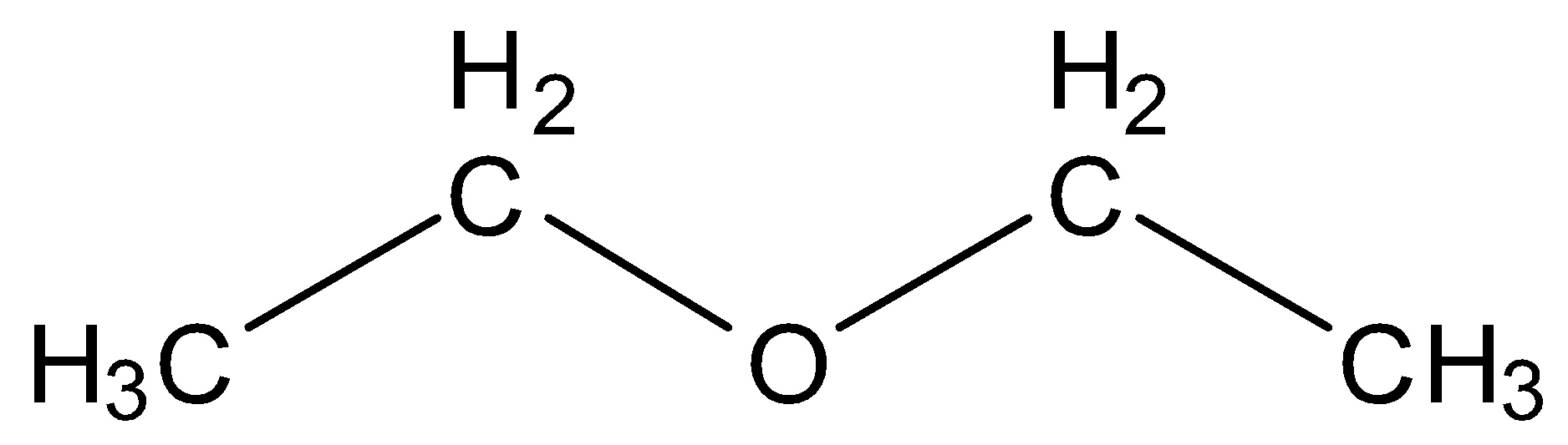
This structure contains four carbon and ten hydrogen which is equal to the molecular formula given in the question but it is still not the correct option as the ether here is symmetrical which is not a condition of answer so incorrect option.
(D) Methoxy propane 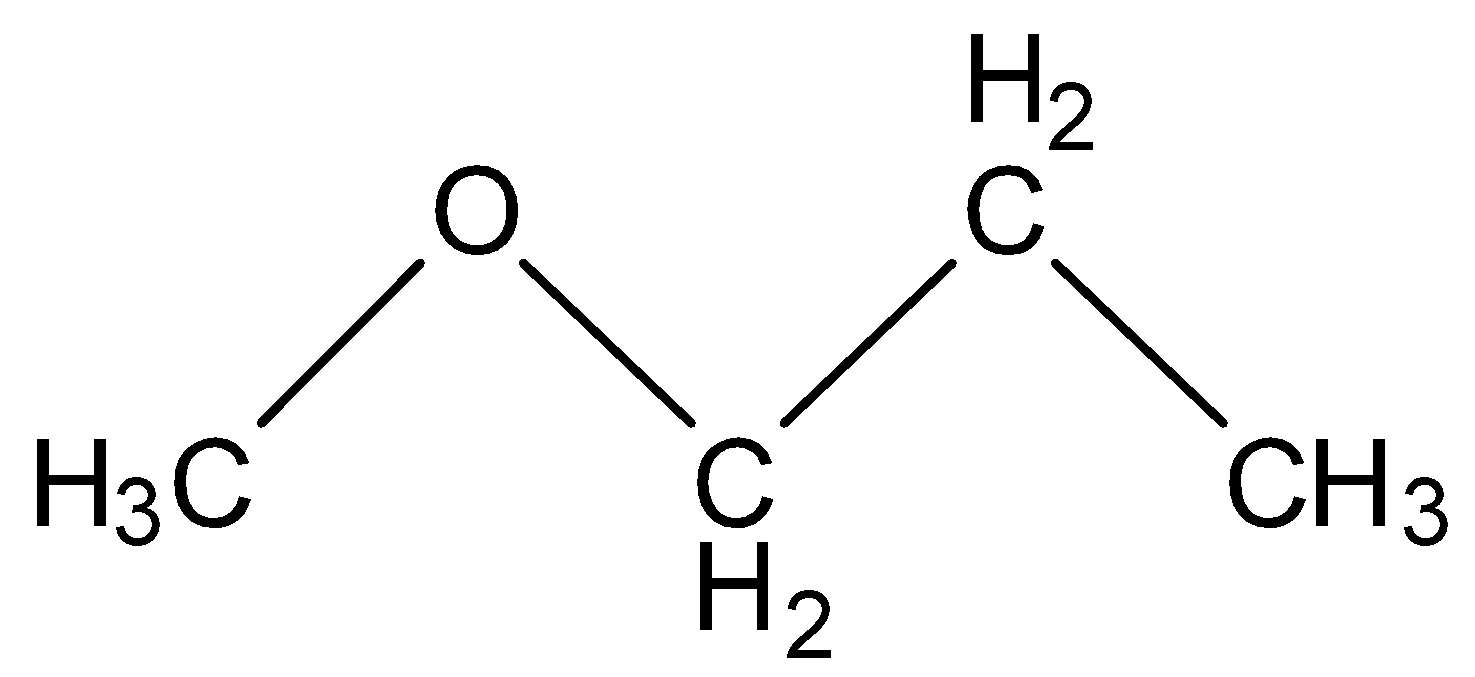
The structure here has ten hydrogens and four carbons and also fulfills the condition of being unsymmetrical so this is the correct option.
So, the correct answer is Option D .
Additional information:
The nomenclature of ether is in the form of alkoxy alkane in which the complex substituent is the root word alkane and less complex substituent is alkoxy. The linkage between carbon oxygen and carbon is bent at an angle of 111∘ and the bond length of carbon oxygen bond is 141 pm.
Note: There is a large electronegativity difference between carbon and oxygen so it is polar in nature and the hydrogens attached to carbon α to oxygen are acidic in nature and can be easily removed.
