Question
Question: The IUPAC name is: 
A. Trimethylamine
B. 2-methyl ethanamine
C. N,N-Dimethylmethanamine
D. Trimethyl ammonia
Solution
We know that amines are basic chemical derivatives of ammonia in which the bonded hydrogens are replaced by an alkyl or aryl groups. Amines are classified into,
Primary amine: One organic group attached to nitrogen atoms.
Secondary amine: Two organic groups bonded to nitrogen atoms.
Tertiary amine: Three organic groups bonded to nitrogen atoms.
Complete step by step answer:
The given compound is,
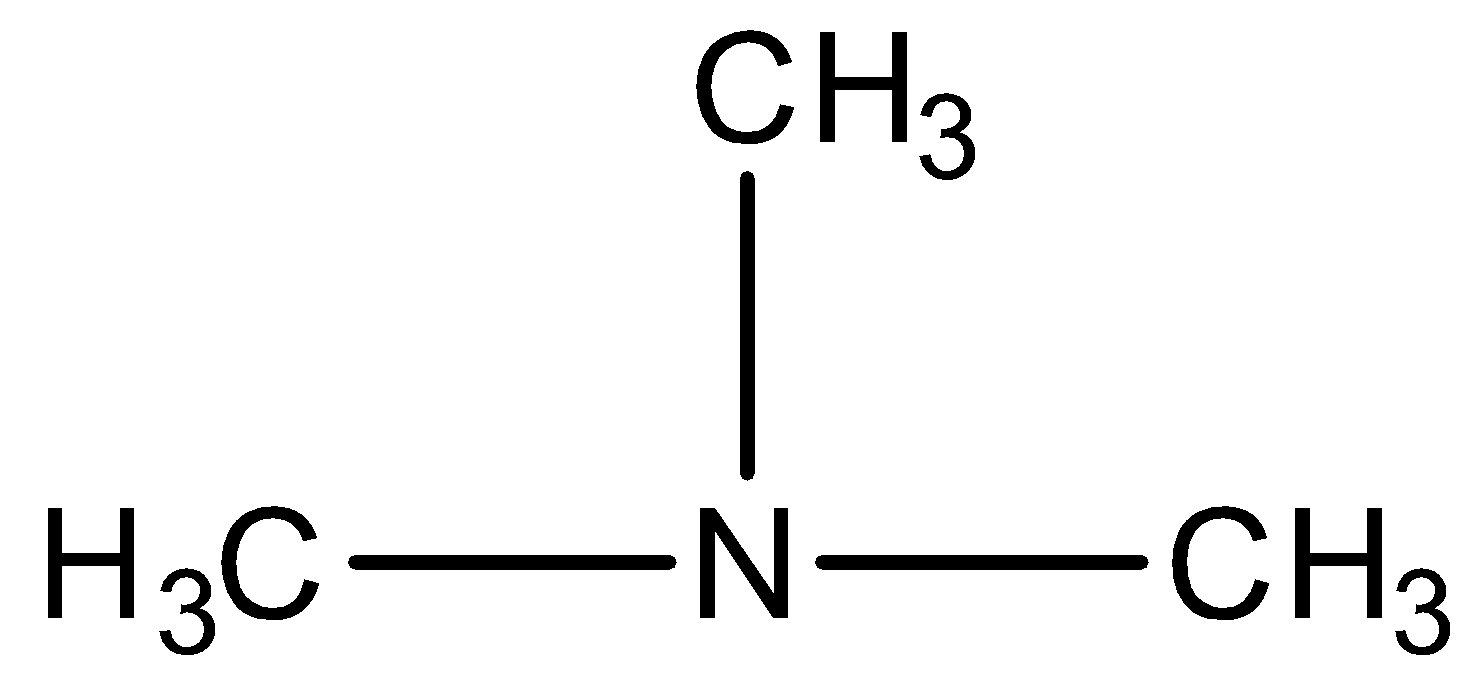
We have to know that all the three hydrogens of ammonia are replaced by alkyl groups and hence it is a tertiary amine (3∘ amine).
The IUPAC name of tertiary amine is given by the set of rules given below:
IUPAC nomenclature of tertiary amine:
The parent compound, that is, the longest alkyl chain is identified.
The parent compound is named as alkanamine.
The other two-alkyl groups are named as N− alkyl groups, and they are used as a prefix in front of the parent alkanamine.
If the two alkyl groups are different, they are listed sequentially.
If the two groups are identical, they are written as N− dialkyl.
In the given compound, the longest alkyl chain is methane; therefore the parent compound is methanamine. There are two methyl groups present and they are identical. So, the compound is named as N,N-dimethylmethanamine.
So, the correct answer is Option C.
Note: We can write the molecular formula of trimethylamine as C3H9N. We have to know that trimethylamine is colourless gas that has fishlike smell at lower concentrations. They produce harmful oxides of nitrogen when they undergo combustion. The conjugate base of trimethylamine is trimethylammonium.
