Question
Question: The IUPAC name for the compound is: 
Solution
The compound contains both alkene and keto groups. The parent functional group of this compound is ketone since this functional group gets the higher priority compared to alkene. Thus, the parent name of the compound will get the name ketone so the suffix will be “one”.
Complete step by step answer:
The general formula for alkene isCnH2n. The suffix ‘-ene’ is used to indicate the alkene group. The longest chain is chosen in such a way that both the carbon atoms of the double bond are included.
When both the carbonyl group (or keto group) takes the precedence than the double bond. While naming the ‘en’ suffix follows the parent chain directly. The location of the double bond is/are indicated before the parent name. Always we need to indicate the carbonyl position between the ‘-en’ and ‘-one’ suffixes.
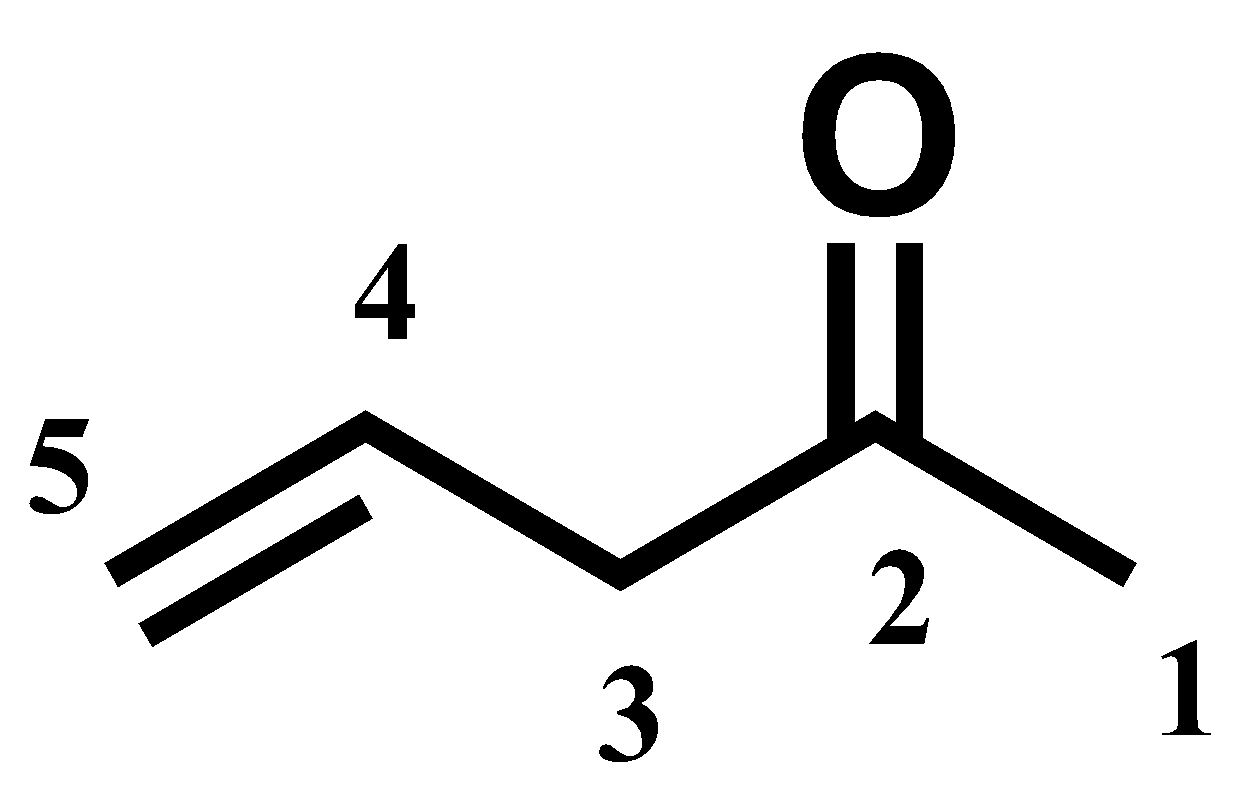
Hereby, we have numbered the compound that satisfies the IUPAC nomenclature.
Since it is a 5 carbon atoms chain, the alkene is named as pentene. The carbonyl group is indicated by the suffix ‘-one’. Thus, the name becomes “pentanone” (an -e’ is left for convenience).
Now the location of alkene and carbonyl groups must be specified. Carbonyl group is in 2nd position and alkene double bond starts at the 4th carbon. The name of the compound is pent-4-en-2-one.
So, the IUPAC name of the given compound is Pent-4-en-2-one.
Note:
Try to keep in mind the following points to avoid mistakes:
1.The primary functional must be given preference while numbering. Following numbering is wrong where the alkene group gets the preference.
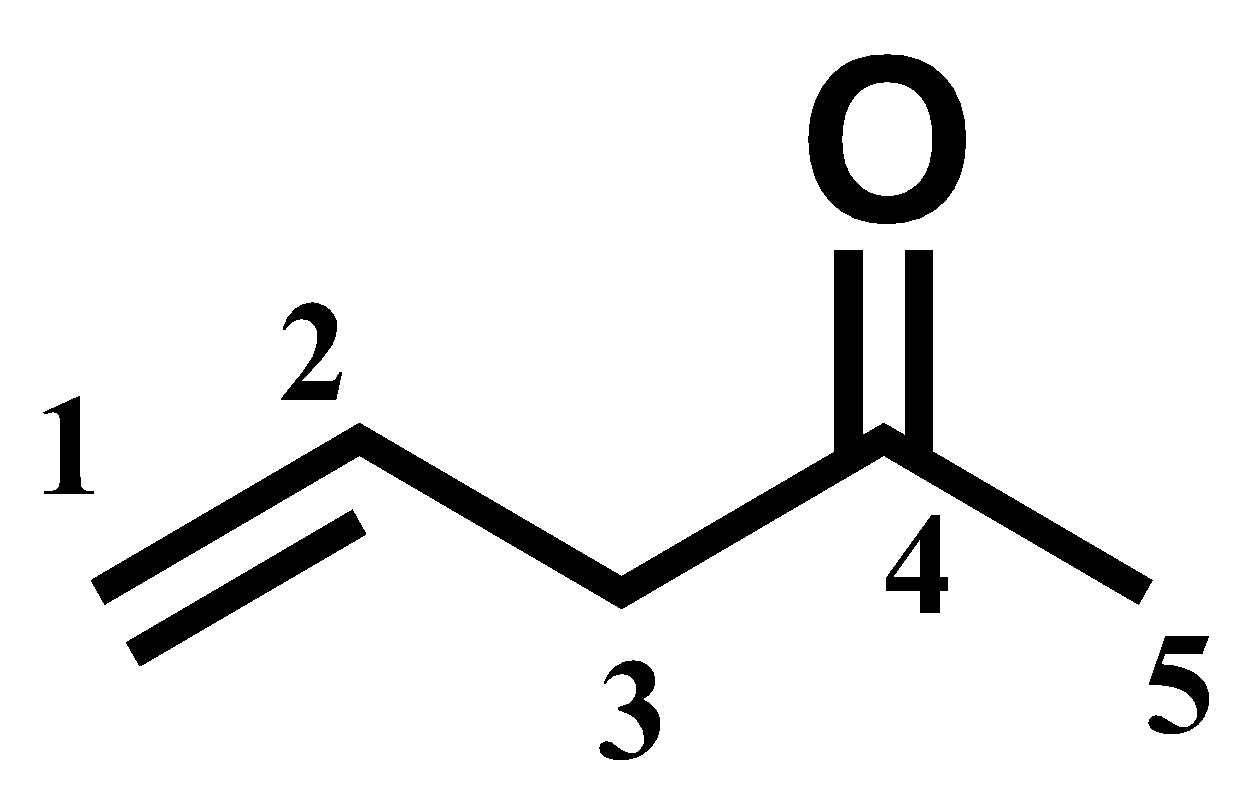
2.Always make sure while numbering the parent chain, the substituents, if any present, should get the lowest number.
3.If more than one double bond is present the compound is named as a diene, triene or equivalent prefix indicating the number of double bonds, and each double bond is assigned a locator number.
