Question
Question: The isomer of propyne is: A. Allene B. Propene C. Cyclopropane D. Propane...
The isomer of propyne is:
A. Allene
B. Propene
C. Cyclopropane
D. Propane
Solution
The compounds that have the same molecular formula but different structures (differ in physical and chemical properties) are called isomers. In propyne the two carbon atoms have sp hybridization.
Complete step by step answer:
To understand this question we should know about functional isomerism- when two more substances with the same molecular formula but different functional groups are functional isomers.
Now we already know that propyne
CH3−C=CH
In this α carbon atoms have sp hybridization and one carbon has sp3
look at the hybridization of each option given-
allene - CH2=C=CH2
In this the central carbon is sp-hybridized and the two terminal carbon atoms are sp2 hybridized.
Propene - CH2=CH−CH3
In this the two carbon atoms are sp2 and one carbon atom is sp3 hybridized.
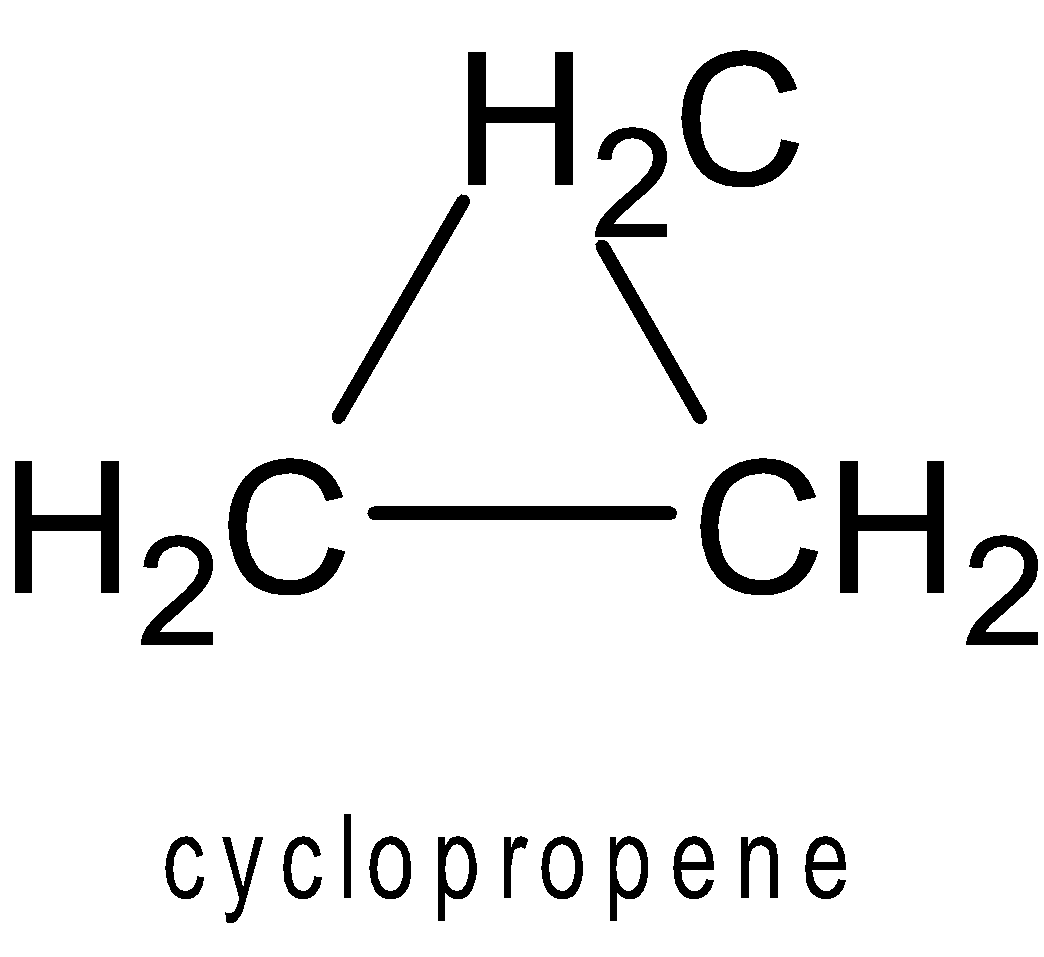
Cyclopropane-
It is also sp3 hybridized
Propane – CH3−CH2−CH3
In this all bonds are single, hence it undergoes sp3 hybridization.
So we can conclude that allene (CH2=C=CH2) is a structural isomer of propyne because it has same molecular formula but different functional groups.
So, the correct answer is Option A.
Note: Isomers have different arrangement of atoms due to molecular rotating. Molecules having the same molecular formula have different isomers due to arrangement of atoms. Functional isomerism occurs when substances have the same molecular formula but different functional groups. Proyne can also show ring chain isomers called cyclopropene.