Question
Question: The ionic equivalent conductivity of $C_2O_4^{-2}$, $K^+$ and $Na^+$ ions are $x, y, z \ S \ cm^2 \ ...
The ionic equivalent conductivity of C2O4−2, K+ and Na+ ions are x,y,z S cm2 eq−1 respectively. Then, Λeq∘ of (NaOOC−COOK) is
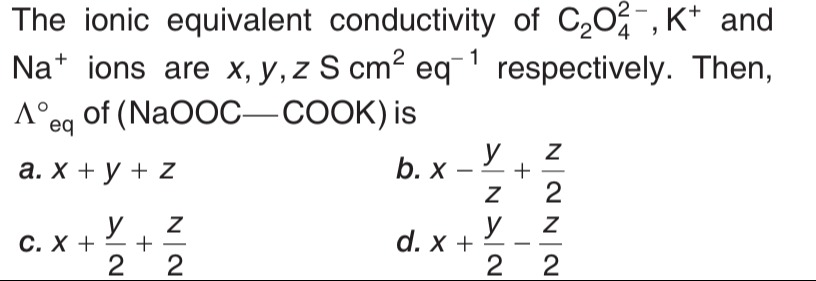
x+y+z
x−zy+2z
x+2y+2z
x+2y−2z
x+2y+2z
Solution
The salt is (NaOOC−COOK), which is Potassium Sodium Oxalate, with the chemical formula NaKC2O4. When this salt dissolves in water, it dissociates into ions:
NaKC2O4→Na++K++C2O42−
According to Kohlrausch's Law of Independent Migration of Ions, the equivalent conductivity of an electrolyte at infinite dilution (Λeq∘) is the sum of the equivalent conductivities of the ions produced by one equivalent of the electrolyte.
Λeq∘(electrolyte)=∑(number of equivalents of ion per equivalent of electrolyte)×λeq∘(ion)
Alternatively, and more commonly applied:
Λeq∘(electrolyte)=λeq∘(total cations)+λeq∘(total anions)
where λeq∘(total cations) is the sum of the equivalent conductivities of all cations produced from one equivalent of the electrolyte, and similarly for anions.
Given:
-
λeq∘(C2O4−2)=x S cm2 eq−1
-
λeq∘(K+)=y S cm2 eq−1
-
λeq∘(Na+)=z S cm2 eq−1
Applying Kohlrausch's Law:
Λm∘(NaKC2O4)=λm∘(Na+)+λm∘(K+)+λm∘(C2O42−)
Using λm∘(Izi)=∣zi∣λeq∘(Izi):
λm∘(Na+)=1×z=z
λm∘(K+)=1×y=y
λm∘(C2O42−)=2×x=2x
Λm∘(NaKC2O4)=z+y+2x
The relationship between molar and equivalent conductivity is Λm∘=zΛeq∘, where z is the total charge per formula unit. For NaKC2O4, z=2.
Λeq∘(NaKC2O4)=2Λm∘(NaKC2O4)=2z+y+2x=2z+2y+x
Λeq∘(NaKC2O4)=x+2y+2z
