Question
Question: The intermetallic compound LiAg crystallizes in a cubic lattice in which lithium and silver atoms ha...
The intermetallic compound LiAg crystallizes in a cubic lattice in which lithium and silver atoms have coordination numbers of 8. To what crystal class does the unit cell belong?
A. Simple cubic
B. Face-centred cubic
C. Body-centred cubic
D. Edge-centred cubic
Solution
Hint Two different metals react each other and form a compound which contains both the metals called intermetallic compounds. The properties of the intermetallic compounds resemble the properties of the individual metal atoms.
Complete step by step answer:
- In the question it is given that LiAg crystallizes in a cubic lattice.
- In LiAg crystal lithium and silver have a coordination of 8. We have to find under which crystal structure it comes among the given options.
- In the question itself it is given that the coordination number of lithium and silver atoms is 8.
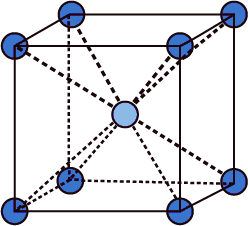
- From the above structure only we can say that each atom will have the coordination of eight.
- The central atom is going to coordinate with eight atoms which are present at the corners of the cube.
- Therefore LiAg crystal belongs to the BCC (body - centered Cubic lattice) unit cell.
- So, the correct option is C.
Note: Sodium chloride crystal belongs to the category face-centered cubic lattice structure due to the presence of sodium atoms at the middle of the faces in the cube. While coming to bcc (body - centered Cubic lattice) the metal atom is going to present in the middle of the cube with a coordination of eight.
