Question
Question: The indicated atom is not a nucleophilic site in: A. 
B.
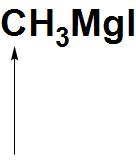
C.
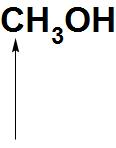
D.
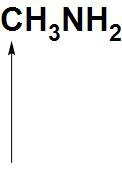
Solution
As we know that nucleophiles are chemical species that donates an electron pair to form a chemical bond. As these can donate electrons these are also called as lewis bases. These undergo nucleophilic addition and nucleophilic substitution reactions.
Complete step by step answer:
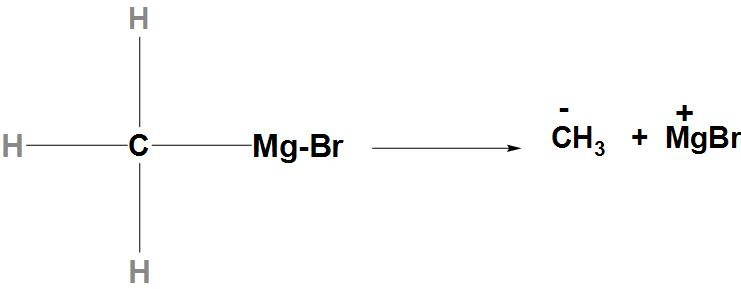
- Let’s first discuss CH3MgI: CH3MgI is also called a Grignard reagent. When the bond between C-Mg breaks there is formation of carbanion that takes place, that has negative charge and can donate an electron pair. Hence, we can say that the indicated atom that is carbon atom is a nucleophilic site in CH3MgI.
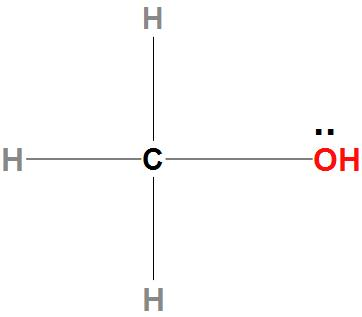
- Now, let see about CH3OH: We can see here that there is a lone pair of electrons present on oxygen atoms. Hence, it can donate an electron pair. Hence, we can say that the indicated oxygen atom is a nucleophilic site in CH3OH.
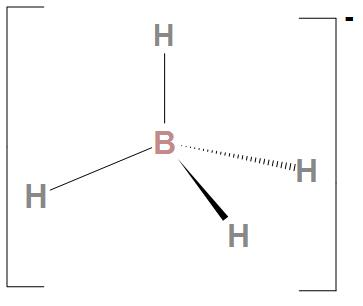
- Now, let see about BH4−: We can see here that there is no lone pair of electrons present on boron atoms. Hence, it can’t donate an electron pair. Hence, we can say that the indicated atom that is boron atom is not a nucleophilic site in BH4−.
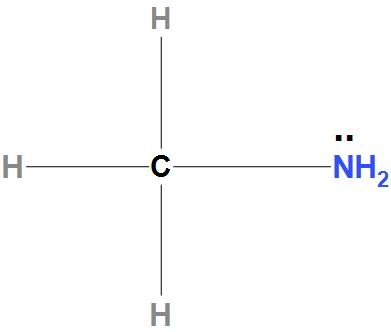
- Now, let see about CH3NH2: We can see here that there is a lone pair of electrons present on nitrogen atoms. Hence, it can donate an electron pair. Hence, we can say that the indicated atom that is a nitrogen atom is a nucleophilic site in CH3NH2.
Note: - We should not get confused in the terms electrophile and nucleophile. The main difference in between both these is nucleophile is negatively charged/neutral and also called as lewis base. These are electron rich species that donate a pair of electrons to form a covalent bond.
- Whereas, electrophile is positively and also called as lewis acids. These are electron deficient species that accept a pair of electrons to form a covalent bond.
