Question
Question: The increasing order of reactivity of the following compounds towards aromatic electrophilic substit...
The increasing order of reactivity of the following compounds towards aromatic electrophilic substitution reaction is:
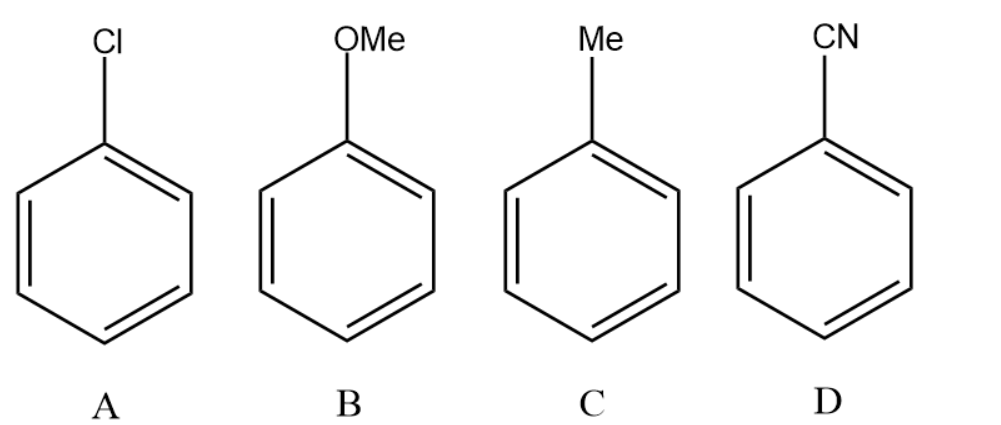
(A) D < B < A < C
(B) D < A < C < B
(C) A < B < C < D
(D) B < C < A < D
Solution
It is important to understand the concept of electrophilic aromatic substitution and the nature of attacking ions. Electrophiles are attracted to the aromatic system when nucleophilicity of the system is increased. This means that charge density should increase in the aromatic system. Identify if the groups withdraw electrons or donate electrons and thereby determine the order of reactivity.
Complete step-by-step answer:
Electrophilic aromatic substitution also called E.A.S is an organic reaction in which an atom gets attached to the present aromatic system and replaces the hydrogen atom present. Some of the most important and known Electrophilic aromatic substitution reactions are:
- Aromatic nitration
- Aromatic halogenation
- Aromatic sulfonation
- Friedel-Crafts acylation
- Friedel-Crafts alkylation
In the above set of compounds, we find the following functional groups attached to the aromatic system:
(A) Chlorine atom : Mild deactivating group. Reduces the nucleophilicity as it withdraws electrons from the aromatic system.
(B) Methoxy group : Activating group. Increases the nucleophilicity as it donates electrons to the aromatic system.
(C) Methyl group : Activating group. Increases the nucleophilicity as it donates electrons to the aromatic system.
(D) Nitrile group : Strong deactivating group. Reduces the nucleophilicity as it withdraws electrons from the aromatic system. Based on the above explanation we conclude that the increasing order of reactivity of the following compounds towards aromatic electrophilic substitution reaction is:
D < A < C < B.
Therefore, the correct answer is option (B).
Additional information: Electronic factors are the factor that influence various organic reactions and rearrangements. Electronic effects are significantly observed in organic aromatic compounds.
Electronic factors are:
- Inductive effect
- Resonance
- Mesomeric effect
- Electromeric effect
- Hyperconjugation.
Note: It is important to understand why methoxy group is given higher priority than methyl group when deciding the order. Methoxy group increases charge density through resonance. On the other hand, methyl groups increase charge density by hyperconjugation. In electrophilic aromatic system reactions, resonance is given higher priority than hyperconjugation. Thus the compound methoxy group activates the system better than the methyl group.
