Question
Question: The increasing order of \(p{{K}_{b}}\) for the following compounds will be: (a) \(N{{H}_{2}}-CH=NH...
The increasing order of pKb for the following compounds will be:
(a) NH2−CH=NH
(b)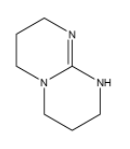
(c) CH3−NH−CH3
Solution
First we have to find the compound which has the highest basic strength because the compound with highest basic strength will have the highest Kb value and lowest pKband vice-versa as Kb=pKb1. Now solve it.
Complete step by step solution:
To find out the compound which has the highest value of pKb, we should know about the basic strength of that compound. The compound which has the highest basic strength , will have the highest Kbvalue and thus, have the lowest value of pKb and vice-versa. It is so because pKb is inversely proportional to Kb as;
Kb=pKb1
So, thus one having a higher value of Kb, will have a lower value of pKb and hence, the lowest basic strength.
The basic strength of the compound depends:
1. On the presence of the number of electron donating groups in that very compound.
2. The lone pair or the electron pairs of the donating group should not be in resonance or conjugation.
Now, considering the statement ;
In option (a), there are two −NH2 groups, but the lone pairs on nitrogen atoms are conjugated. So, its basic strength decreases and has lesser Kb value.
In option (b),there are three electron donating nitrogen groups containing lone pair of electrons which are not neither in conjugation nor in resonance with the each other and hence it has the highest basic strength among all and has the largest Kb value.
In option (c), there is one −NH2 group, but the lone pair on the nitrogen atom is in conjugation. So, its basic strength decreases and has lesser Kb value than the option (b) and even from option (a) in which there are two −NH2 groups.
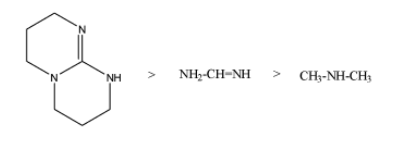
So, the increasing order of the Kb value is as;
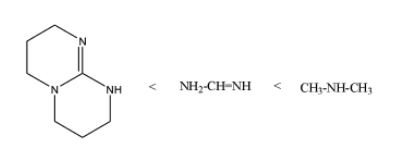
Then, the increasing order of pKbis as;
Note: The electron withdrawing group if attached to the compound, it decreases the basicity as it withdraws the electrons from the compound and its basic strength decreases and hence, have lower Kb values and higher pKb values.
