Question
Question: The increasing order of nucleophilicity of the following nucleophiles is (1) \(C{{H}_{3}}CO_{2}^{...
The increasing order of nucleophilicity of the following nucleophiles is
(1) CH3CO2−
(2) H2O
(3) CH3SO3−
(4)OH−
(a) (1)<(4)<(3)<(2)
(b) (2)<(3)<(1)<(4)
(c) (4)<(1)<(3)<(2)
(d) (2)<(3)<(4)<(1)
Solution
Nucleophiles are the nucleus loving species and have a strong tendency to give the electrons to other species. If you know the rules of the nucleophilicity of a nucleophile, then you can easily arrange the given nucleophiles in their increasing order. Now solve it.
Complete answer:
First of all, let’s discuss what nucleophiles are. Nucleophiles are those substances i.e. the compounds or the elements which have a strong tendency to donate the electrons and are nucleus loving species.
The conditions for the nucleophilicity of a species are as ;
1.Neutral nucleophiles have weaker nucleophilicity than the negatively charged nucleophiles and thus, are weak nucleophiles.
2.More stable is the negative charge on the nucleophile, lesser is the nucleophilicity of the species and vice-versa.
3.More is the resonating structures of the species; more is the stability of that very species and lesser is its nucleophilicity and vice-versa.
Now considering the statement as
(1) CH3CO2−
Its structure of is as-
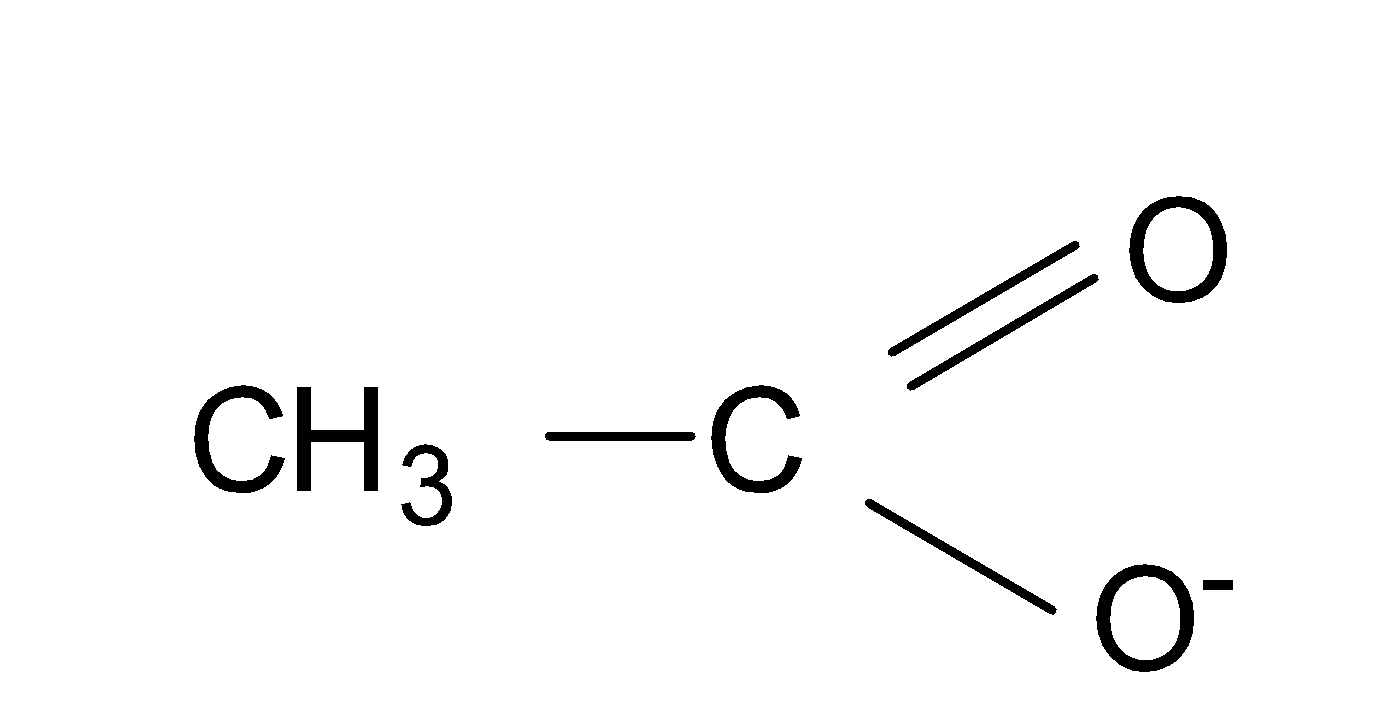
In this, the negative charge is stabilized due to the resonance and delocalization of the electrons takes place which leads to the stability of the compound and it forms three resonating structures.
(2) H2O
The structure of water is as-
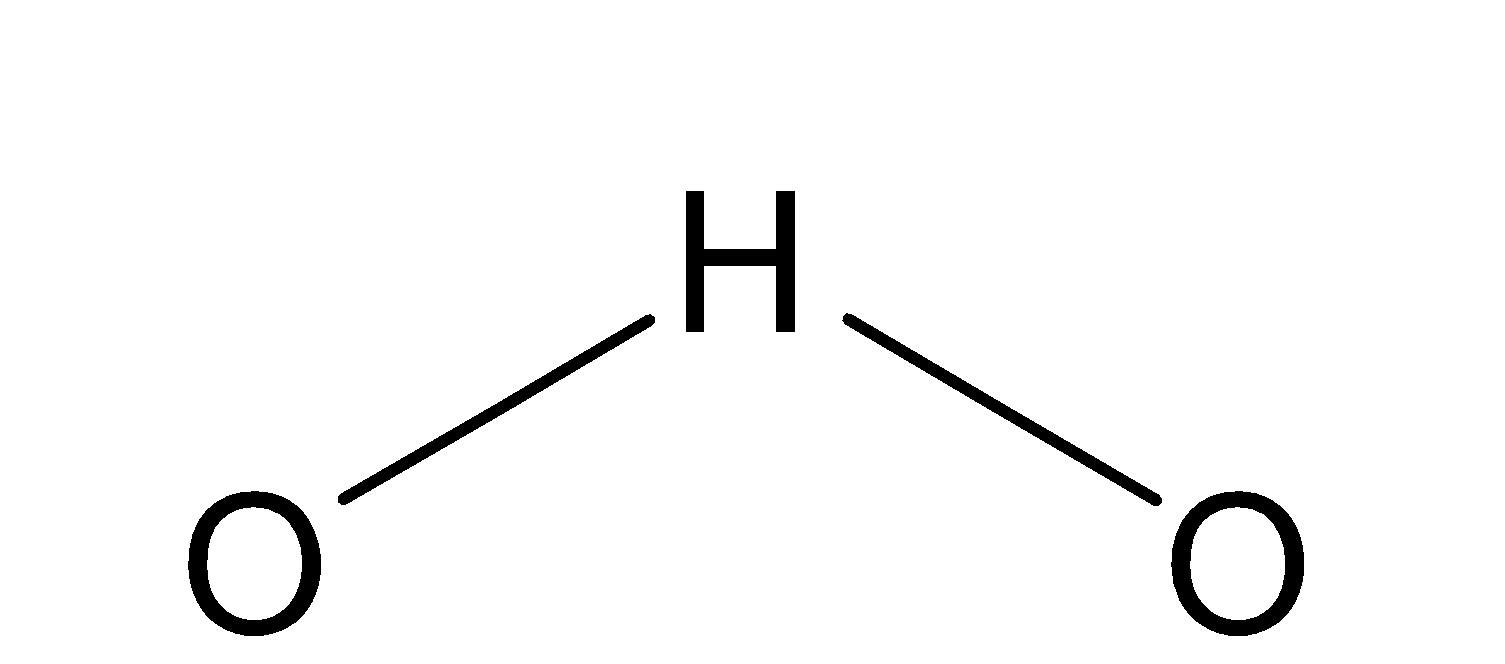
Water is neutral because it has no charge on it and thus, has least nucleophilicity.
(3) CH3SO3−
The structure of is as-
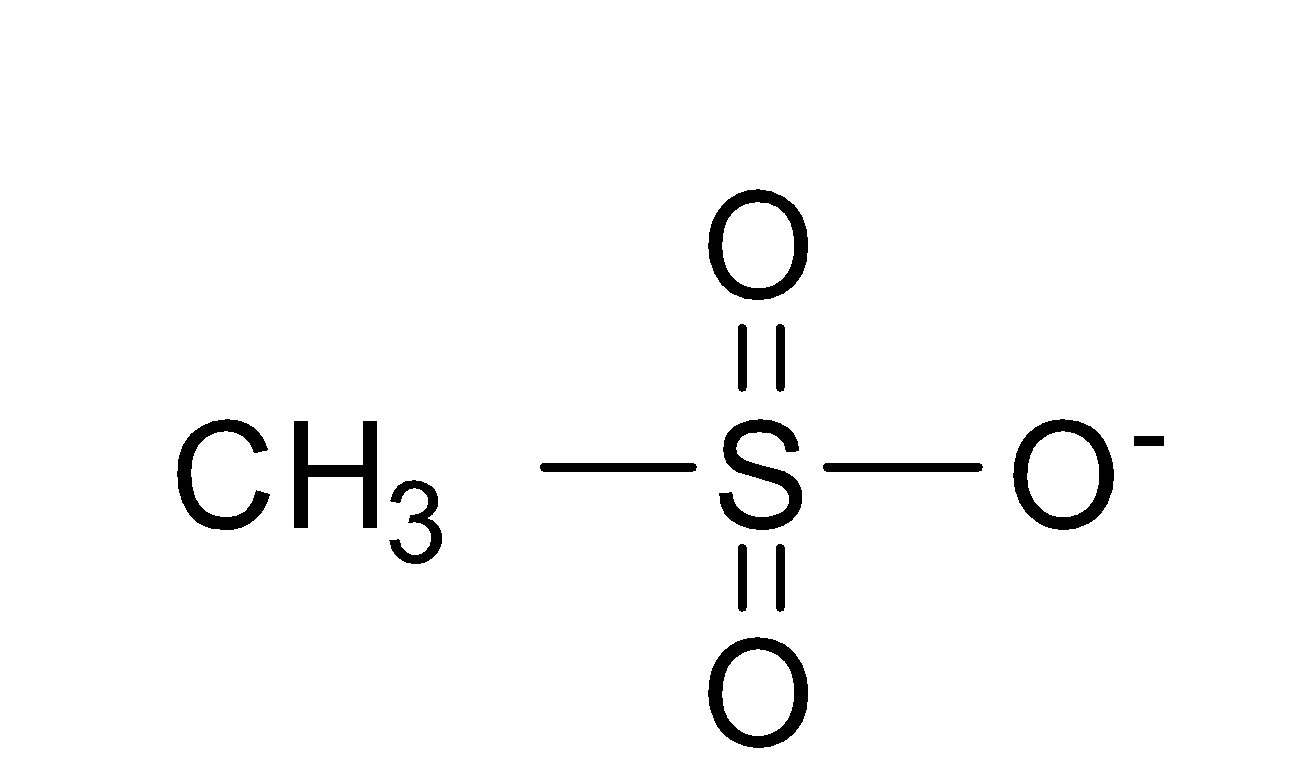
In this negative charge is stabilized due to the resonance and delocalization of the electrons takes places which leads to the stability of the compound and it forms four resonating structures and thus, the negative charge is very stable and thus, is weaker nucleophile than the
(4)OH−
In this, there is no resonance and hence negative charge is not stabilized and thus, has the highest nucleophilicity than all the nucleophiles.
So, therefore, the increasing order of the nucleophiles as;
H2O<CH3SO3−<CH3CO2−<OH−
i.e. (2) < (3) < (1) < (4)
Hence, option (b) is correct.
Note:
Don’t get confused in the electrophiles and the nucleophiles. Electrophiles are the species which are electron loving and have the tendency to accept the electrons whereas on the other hand, nucleophiles are the species which are nucleus loving and have the tendency to donate the electrons.
