Question
Question: The increasing order of nucleophilicity of the following nucleophiles is: \((1)\) \({{C}}{{{H}}_3}...
The increasing order of nucleophilicity of the following nucleophiles is:
(1) CH3CO2− (2) H2O (3) CH3SO3− (4) −OH
(a) 1<4<3<2
(b) 2<3<1<4
(c) 4<1<3<2
(d) 2<3<4<1
Solution
The order of nucleophilicity and basicity need not be the same. Carefully analyse the given species and identify the donor atoms in each of the species. Find a relation between the donating ability and nucleophilicity of each of the given compounds.
Complete step by step answer:
Electron-rich species or electron donors are called nucleophiles. Nucleophiles can be classified into three categories:
Charged nucleophiles - Nucleophiles which are negatively charged. Examples: H− , −OH , X− etc.
Neutral nucleophiles – Nucleophiles which do not carry a charge. Example:
Ambident nucleophiles – Species having two nucleophilic centres out of which one is neutral and another is charged. Example:
Nucleophilicity is a steric sensitive quantity which means that the nucleophilicity decreases as the size increases because when the size increases, it becomes more and more difficult for it to approach the electrophiles.
Nucleophilicity and basicity depend upon the availability of a lone pair of electrons. A nucleophile donates its lone pair of electrons to a hydrogen atom but when it is donated to a carbon, it is called a base.
If the nucleophilic atom or the centre is the same, nucleophilicity parallels basicity, i.e., as the basicity increases, the strength of the nucleophile also increases.
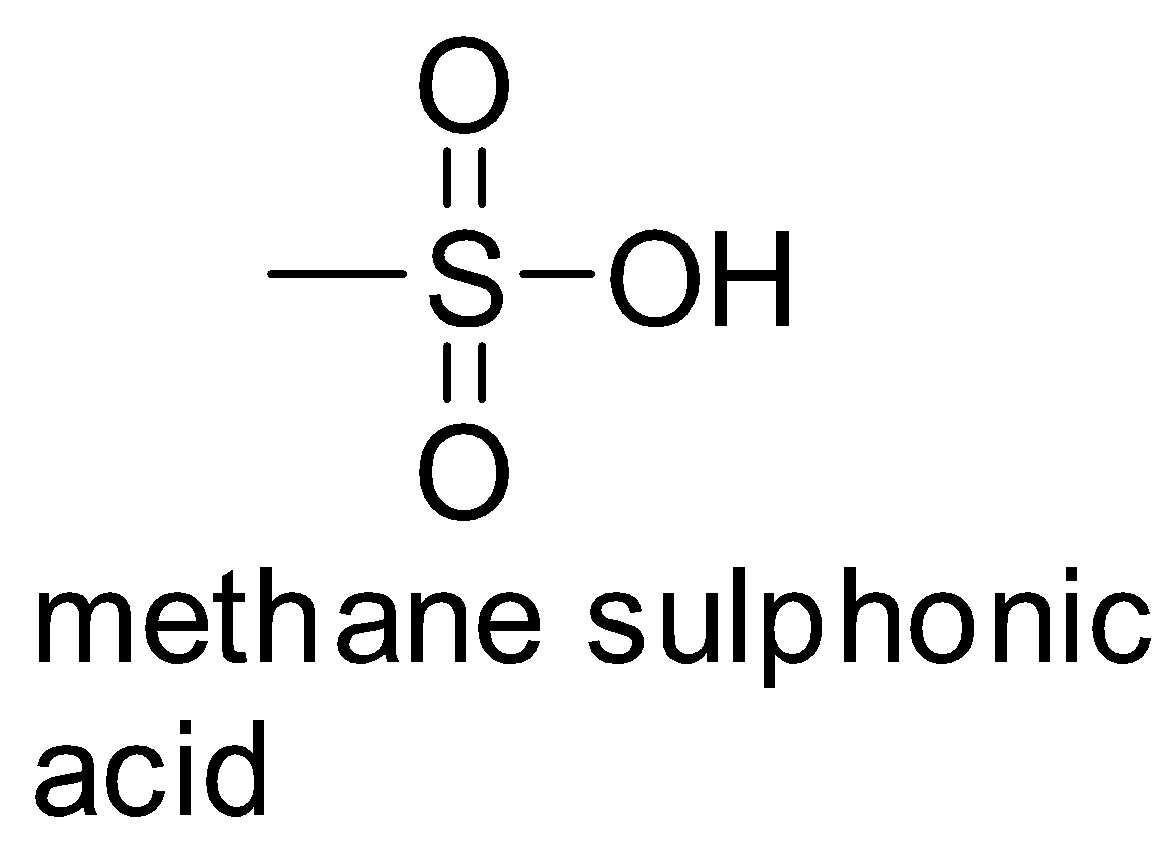
The basicity of these can be found out by looking at their acidities. We know that stronger the acid, the weaker will be its conjugate base.
So, compound (3) is derived from the acid, methane sulphonic acid which is a comparatively stronger acid than acetic acid. Compound (1) is derived from acetic acid. So, this indicates that the basicity of compound (3) is less than compound (1) .
−OH is the strongest base in the given set of compounds and H2O is the weakest base, because the conjugate acid of −OH is H2O and that of H2O is . The tendency will be to quickly get rid of a proton so that it can exist as a water molecule. So, H2O is the weakest base.
Therefore, the order of basicity will be:
H2O < CH3SO3− < CH3CO2− < H2O
Hence, option (b) 2<3<1<4 is the correct answer.
Note:
Nucleophilicity and basicity are not the same. The similarity between them is that both nucleophiles and bases are electron rich and have good donor ability. But the difference is that bases love protons, i.e., they abstract protons from wherever they can, whereas, nucleophiles love electron deficient species ( electrophiles ) .
