Question
Question: The increasing order of basicity of the compounds is: (a)  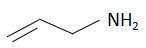
(b) 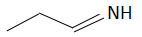
(c) 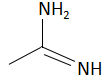
(d) 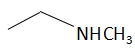
A. (b) < (a) < (c) < (d)
B. (b) < (a) < (d) < (c)
C. (d) < (b) < (a) < (c)
D. (a) < (b) < (c) < (d)
Solution
The basic nature of any compound depends upon the electron donating tendency of the atom having a lone pair of electrons. The basic nature is also affected by certain other factors such as electronegativity, presence of electron donating or electron withdrawing groups around the atom, etc.
Complete step by step answer:
The order of basicity or basic nature depends on how easily an atom can donate the lone pair of electrons or loses the electrons or in simple terms, the electron donating tendency of the atom. In compound (b) the nitrogen atom is sp2 hybridized, so it is the least basic among all the given compounds. Thesp2 hybridized carbon has a greater electronegativity and electrons present on an electronegative atom are not easy to donate. Compound (c) is a very strong nitrogenous organic base as the lone pair of electrons on one nitrogen atom delocalized with the help of resonance. This makes another nitrogen atom negatively charged and the resultant conjugate acids have two equivalent resonating structures. Thus, it is most basic in the given compound. Compound (d) is a secondary amine and is more basic than the compound (a) which is a primary amine.
Therefore, the increasing order of basicity is: B. (b) < (a) < (d) < (c)
So, the correct answer is Option B.
Note:
The greater the availability of electrons on the atom, the more is the tendency of the atom to donate it. An electron withdrawing group decreases the energy density on the atoms and an electron donating group increases the electronic density over the atoms. Thus, the electron donating tendency will be:
Tertiary amine > Secondary amine > Primary amine
